Question
Question: In the following reaction sequence, the amount of D(in g) formed from 10 moles of acetophenone is: ...
In the following reaction sequence, the amount of D(in g) formed from 10 moles of acetophenone is:
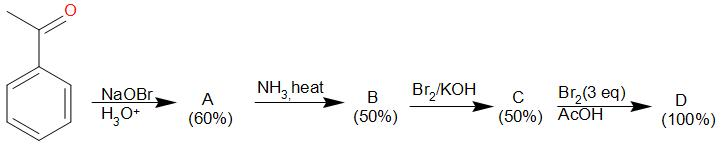
Solution
In a carbonyl group, a more negative oxygen atom is bonded to a less electronegative carbon atom. This difference in electronegativity draws electron density away from the carbon atom making it an electrophile. As a result, carbonyl compounds are susceptible to nucleophilic attacks.
Complete step by step answer:
Acetophenone is a methyl ketone.
Methyl ketones when reacted to sodium hypohalite are oxidised to form sodium salts of corresponding carboxylic acid, along with haloform.
Acetophenone is oxidized by sodium hypobromite to sodium benzoate which in acidic medium forms benzoic acid. A is benzoic acid.
The yield of reaction is given to be 60%. So, 10 moles of acetophenone will give,
10060×10=6moles of benzoic acid.
Benzoic acid when treated with ammonia forms ammonium benzoate which on heating, loses a water molecule to form benzamide. B is benzamide.
We have 6 moles of benzoic acid and yield of the reaction is given to be 50%. As a result, we obtain 3 moles of benzamide.
Benzamide in reaction with bromine in presence of potassium hydroxide gives Hoffmann Bromamide reaction which is a stepping down reaction and a carbon atom is lost. The product obtained is aniline. C is aniline.
We have 3 moles of benzamide and the yield of reaction is 50%. Thus, 1.5 moles of aniline are obtained.
Aniline when exposed to 3 equivalents of bromine in presence of ethanoic acid, undergoes tri-substitution and forms 2,4,6-tribromoaniline.
The yield of the reaction is 100%. Thus, 1.5 molecules of aniline will form 1.5 moles of 2,4,6-tribromoaniline.
The molar mass of 2,4,6-tribromoaniline is 330g/mol.
So, the mass of 1.5 moles of the compound will be,
m=1.5×330 ⇒m=495g
Therefore, the amount of D formed is 495g.
Note:
Aniline is a strongly activated compound. Its ortho and para positions are susceptible to nucleophilic attack because of the +R effect of the lone pair of nitrogen atoms on the benzene ring.
