Question
Question: In the following reaction: \(2 \mathrm { NO } _ { ( \mathrm { g } ) } + \mathrm { Cl } _ { 2 ( \mat...
In the following reaction:
2NO(g)+Cl2( g) 2NOCl(g)
It is observed that equilibrium is not attained and the rate of forward reaction is greater than rate of backward reaction. Which of the following is true for the reaction?
A

B

C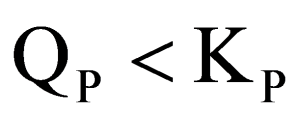
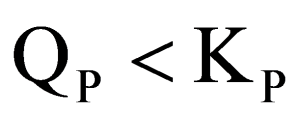
D

Answer
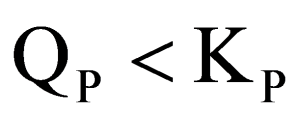
Explanation
Solution
: When Qp<Kp the rate of forward reaction is more
than rate of backward reaction.
