Question
Question: In the following reaction, 
Q is:
(A). 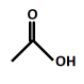
(B). 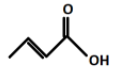
(C). 
(D). 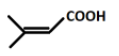
Solution
The reaction of acetone with Ba(OH)2 refers to the condensation reaction between the two reactants molecules. In the reaction the new carbon- carbon bonds formed after the combination of the both reactants molecules. There will be elimination of a simple molecule and then the product formed will react with chlorine and sodium hydroxide to form the product.
Complete step by step answer:
The initial structure is of acetone, having the molecular formula, CH3COCH3 which is a common name for propanone. Another name for acetone is dimethyl ketone. It is used as a solvent in many reactions and it is volatile which means that if it is kept open then it will evaporate.
In the first step acetone reacts with barium hydroxide and forms a product P. When Ba(OH)2 is added to acetone some of the molecules of acetone will undergoes enolization and production of enol will take place. The product formed after the reaction is a β− hydroxy ketone and has 2 functional groups.
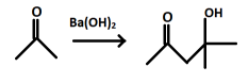
The product P formed will react with Cl2/NaOH and leads to formation of sodium salt of the compound P.
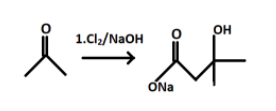
And after hydrolysis the final product formed i.e. Q will be:
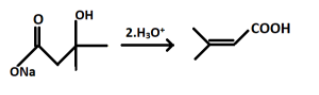
Hence, the correct answer is option (d).
Note: Carbon of the carbonyl group is not reactive towards the electrophilic but oxygen of the carbonyl group will be reactive towards the electrophile. The condensation reaction can take place between two different types of the carbonyl compounds. If the reaction is between two different ketones and aldehydes or ketones then these reactions are known as cross condensation reactions.
