Question
Question: In the following reaction:  is changed into
is changed into 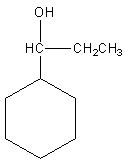 by
by
A. (i) Cu300oC (ii) CH3CH2MgBr,H3+O
B. (i) CrO3 (ii) CH3CH2MgBr,H3+O
C. (i) KMnO4 (ii) CH3CH2MgBr,H3+O
D. (i) Na2Cr2O7+H2SO4 (ii) CH3CH2MgBr,H3+O
Solution
To answer this question we should know the functioning of all the given reagents. The reagents given here in (i), do oxidation of alcohol. The copper is mild whereas the remaining all are strong. The reagent given in (II) is known as Grignard reagent. It is used for alkylation.
Complete solution:
We will check one by one every reagent to obtain the desired product.
A. (i) Cu300oC:
Copper is a mild oxidizing agent. It oxidises the primary alcohol to primary aldehyde and secondary alcohol to ketone.
The reaction of benzyl alcohol with Cu300oC is as follows:
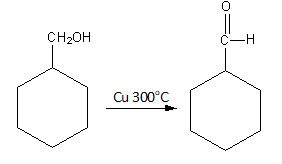
So, benzyl alcohol converts into benzaldehyde by the oxidation caused by copper.
(ii) CH3CH2MgBr,H3+O:
Ethyl magnesium bromide is known as Grignard reagent. This reagent is used for the preparation of alkyl nucleophiles. Ethyl magnesium bromide will give ethyl nucleophile. We know that carbonyl carbon is very electrophilic, so ethyl nucleophiles will attack at carbonyl carbon. The attacks is shown as follows:
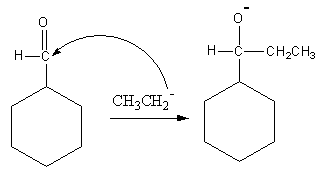
Now the hydronium ion gives protons to the negatively charged oxygen.
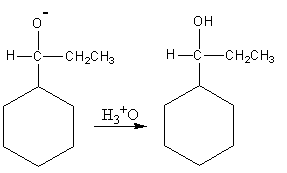
So, the reaction of benzaldehyde with ethyl magnesium bromide gives cyclohexyl propanol which is the desired product. So, option (A) is correct.
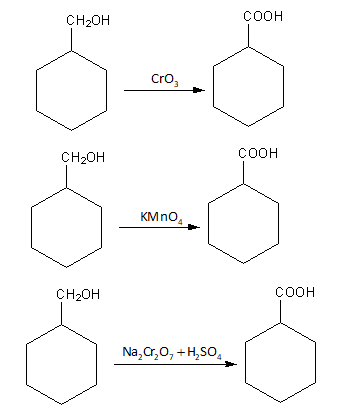
Chromium oxide CrO3, potassium permanganate KMnO4 and sodium dichromate with sulphuric acid Na2Cr2O7+H2SO4, all are strong oxidising agent.
So, the reaction of benzyl alcohol with chromium oxideCrO3, potassium permanganate KMnO4 and sodium dichromate with sulphuric acid gives benzoic acid.
The reactions are shown as follows:
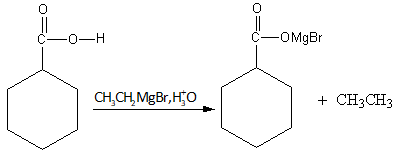
As the ethyl magnesium bromide gives ethyl nucleophile which abstracts the acidic proton from the benzoic acid and gives the benzoate ion and ethane.
So, the reaction of benzyl alcohol with chromium oxideCrO3, potassium permanganate KMnO4 and sodium dichromate with sulphuric acid does not give the desired product.
Hence, option (D) is the correct answer for the given question.
Note: The conversion of alcohol to carbonyl or carboxylic acid is known as oxidation. The reagent cause the oxidation is known as oxidizing agent. Oxidizing agents convert the primary alcohol to carboxylic acid. Mild oxidizing agents convert into carbonyl only. Strong oxidizing agent converts into carboxylic acid. The electron-deficient species is known as electrophile. The electron-rich species is known as nucleophile.
