Question
Question: In the following reaction. The percentage mass of hydrogen in the product (R) is....
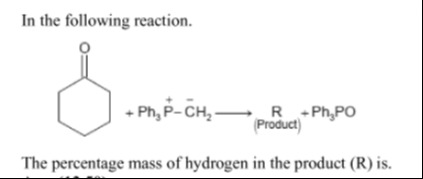
In the following reaction.
The percentage mass of hydrogen in the product (R) is.
Answer
12.5%
Explanation
Solution
The given reaction is a Wittig reaction between cyclohexanone and methylenetriphenylphosphorane (Ph3P=CH2), converting the ketone into an alkene. The product (R) is methylenecyclohexane (C7H12).
To calculate the percentage mass of hydrogen in C7H12:
-
Calculate the molar mass of C7H12:
- Molar mass of C = 12 g/mol
- Molar mass of H = 1 g/mol
- Molar mass of C7H12=(7×12)+(12×1)=84+12=96 g/mol
-
Calculate the mass of hydrogen in one mole of C7H12:
- Mass of hydrogen = 12×1=12 g
-
Calculate the percentage mass of hydrogen:
- Percentage mass of hydrogen =Molar mass of C7H12Mass of hydrogen×100%
- Percentage mass of hydrogen =9612×100%=81×100%=12.5%
Therefore, the percentage mass of hydrogen in the product (R) is 12.5%.
