Question
Question: In the following reaction, ether linkage is cleaved by \(PC{l_5}\) \(R - O - R' + PC{l_5} \to RCl...
In the following reaction, ether linkage is cleaved by PCl5
R−O−R′+PCl5→RCl+R′Cl+POCl3
If C5H12O (ether) forms 2-chloropropane as one of the products, then ether
A) 1-ethoxypropane
B) 2-ethoxypropane
C) 1-methoxybutane
D) 2-methoxypropane
Solution
Given the molecular formula of the ether in the question is C5H12O and when this ether is cleaved byPCl5, we get 2-chloropropane as one of the products. You must know the chemical formula of 2-chloropropane and it is CH3−CH(Cl)−CH3. Do the chemical reaction of C5H12O with PCl5 and examine the products.
Complete step by step solution:
In the question, it is given that ether linkage (R−O−R’) is cleaved by PCl5 as follows:
R−O−R′+PCl5→RCl+R′Cl+POCl3
Now, the molecular formula of the given ether in the question is C5H12O
When C5H12O is cleaved by PCl5, we get 2-chloropropane as one of the products.
As you know, the molecular formula of 2-chloropropane is: CH3−CH(Cl)−CH3. Thus, it has three carbon atoms and seven hydrogen atoms. Consequently, we are left with C2H5 unit from the ether C5H12O, after getting 2-chloropropane as one of the products. Since ether group is R−O−R’, the required R for the given ether will be C2H5 and R’ will be the hydrocarbon unit from 2-chloropropane. Hence, the chemical name of the ether C5H12O will be 2-ethoxypropane.
Now, we can write the required chemical equation between given ether, C5H12O and PCl5 as follows:
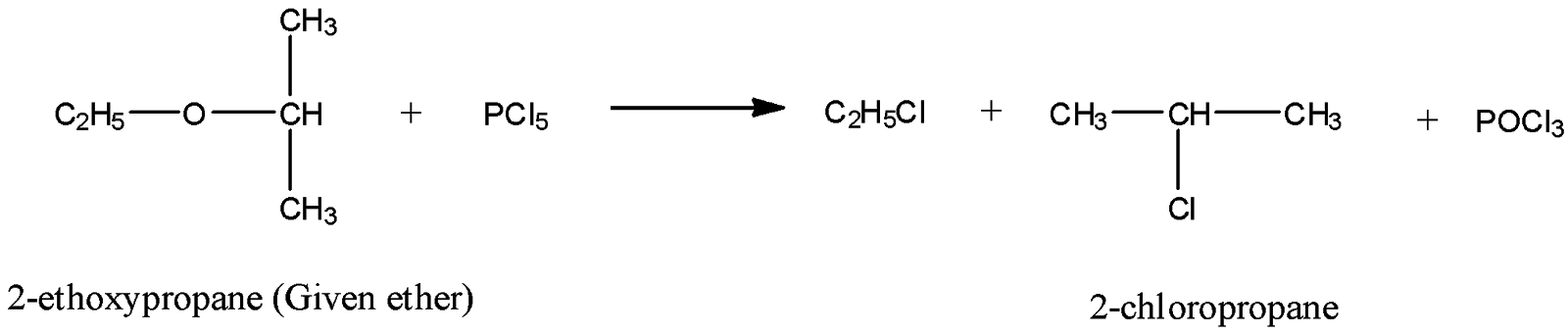
Hence, C5H12O (ether) is 2-ethoxypropane.
Thus, option B is the correct answer.
Note: Phosphorus pentachloride, PCl5 is used as a chlorinating agent. The reaction of cleavage of ethers refers to the chemical substitution reaction. Due to the higher stability of ethers, the cleavage of the C-O linkage is not done easily. Specific reagents are required for cleavage of ethers and PCl5 is that one reagent. It should also be noted from the given chemical reaction in question that dialkyl ethers on reacting with phosphorus pentachloride form alkyl chlorides.
