Question
Question: In the following reaction, compound Z Is: 
A.Benzoic acid
B.Benzaldehyde
C.Acetophenone
D.benzene
Solution
It is a type of an Etard’s reaction which involves the conversion of alkyl benzene or compounds having alkyl group attached to a heterocyclic ring into aromatic aldehydes in presence of chromyl chloride. In this case, we have toluene which reacts with chromyl chloride in the presence of carbon disulfide as a medium forms Etard complex then acidic hydrolysis takes place to form benzaldehyde. This reaction is an Etard reaction.
Complete step-by-step answer: The Etard reaction is one type of reaction in which the direct oxidation of an aromatic or heterocyclic bound alkyl group takes place. In this reaction mechanism, via ene-reaction, the further reaction takes place with the chromyl chloride that forms a precipitate i.e., an Etard complex. The Etard complex which was formed will further decompose by sigma-tropic rearrangement under reducing conditions which prevent the further oxidation to a carboxylic acid. The main reducing condition is provided by a saturated solution of aqueous sodium sulfite (Na2SO3) which prevents further decomposition.
In the aforesaid reaction, the toluene (C6H5CH3) reacts with chromyl chloride in the presence of carbon disulfide as a medium form the Etard complex. This complex gets reduced to in the presence of acidic medium (where acidic hydrolysis takes place) to form benzaldehyde.
In this reaction,to chromyl chloride (CrO2Cl2) attacks on the methyl group connected to the benzene ring to form an intermediate which further undergoes 2−3sigmatropic rearrangement reaction to form a chromium complex, and this complex undergoes hydrolyses so easily to benzaldehyde as a product.
The reaction goes like;
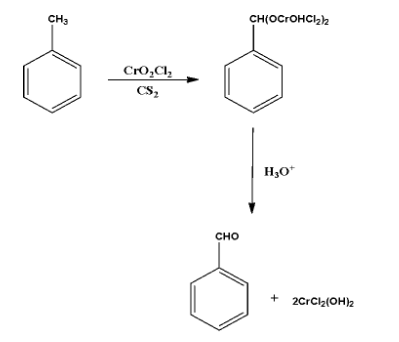
The toluene (C6H5CH3) reacts with chromyl chloride (CrO2Cl2) in the presence of carbon disulfide (CS2) as a medium forms Etard complex which is further reacted with the hydronium ion (H3O+) and form benzaldehyde (C6H5CHO). This reaction is an Etard reaction.
Toluene (C6H5CH3) is oxidized with alkaline potassium permanganate (KMnO4) followed by acidification to form benzoic acid which on further heating with soda-lime that results in the formation of benzene. The oxidation of toluene to benzoic acid (C6H5COOH) and then converted to benzoyl chloride (C6H5COCl) by treating with thionyl chloride (SOCl2) and then treated with methyl lithium (CH3Li) to form Acetophenone (C6H5COCH3) .
So, the correct answer for this question is benzaldehyde formation is taking place.
Therefore, the option B.\;\,\,Benzaldehyde$$$$({C_6}{H_5}CHO) is the correct answer.
Note: The chromyl chloride (CrO2Cl2) is used to form an organic complex Etard Complex which can undergo hydrolysis to form the desired compound. Benzaldehyde (C6H5CHO) can be reduced to toluene (C6H5CH3) by Clemmenson reduction reaction.
