Question
Question: In the following reaction \(C{{H}_{3}}CHO+N{{H}_{2}}.N{{H}_{2}}\to A\xrightarrow{B}C{{H}_{3}}C{{H...
In the following reaction
CH3CHO+NH2.NH2→ABCH3CH3+N2
Identify A and B
(A) CH3CH=NNH2,C2H5ONa
(B) CH3CH2−NH2,C2H5ONa
(C) CH3−NH−NH−CH3,C2H5OH
(D) CH3CH2NH2,C2H5OH
Solution
When aldehydes react with hydrazine, it forms hydrazone. This reacts with base then and hence, ethane is formed.
Complete step by step solution:
The above stated reaction is none other than Wolff-Kishner reduction reaction.
Let us see about Wolff-Kishner reduction at first,
Wolff-Kishner reduction reaction-
This is also known as the nucleophilic addition of hydrazine. It is an organic redox reaction.
Aldehydes or ketones are converted to hydrazine derivatives when reacted with hydrazine. This then reacts with a strong base and heat is provided to form alkane as a product.
In short aldehydes or ketones are converted to corresponding alkanes by this reduction reaction. Mostly, the base used here will be strong so that it would make reduction easy.
Mechanism-
1. Condensation of hydrazine with aldehyde or ketone
2. Formation of hydrazone
3. Deprotonation of hydrazone by strong base
4. Formation of alkyl diimide as an intermediate
5. Formation of alkyl anion by the loss of N2 from alkyl diimide
6. Protonation of alkyl anion by solvent to give alkane.
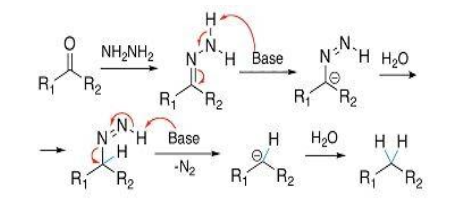
Illustration-
Given reaction is,
CH3CHO+NH2.NH2→ABCH3CH3+N2
Solving this by the mechanism of Wolff-Kishner reaction we get,
1. When acetaldehyde group reacts with hydrazine, hydrazone is formed i.e. CH3CH=NNH2
2. CH3CH=NNH2 reacts with strong base (here, sodium ethoxide) to give corresponding ethane.
Thus, A and B in the given reaction is CH3CH=NNH2,C2H5ONa respectively.
Therefore option (A) is correct.
Note: This reaction requires highly basic conditions. Thus, it is impossible for the base sensitive substrates. Also, in some cases due to steric hindrance in the carbonyl group, formation of hydrazone will not occur.
Option (C) and (D) would never be the answer as we require a strong base for the reaction and these options have ethanol which is unpreferable.
