Question
Question: In the following reaction \({{(C{{H}_{3}})}_{2}}CHC{{H}_{2}}C{{H}_{3}}\xrightarrow[hv]{C{{l}_{2}}}...
In the following reaction
(CH3)2CHCH2CH3Cl2hvN isomers of C5H11Cl.
C5H11Clfractional distillationn distilled products.
The value of N and n are, respectively
A. 6, 6
B. 6, 4
C. 4, 6
D. 4, 4
Solution
. Generally alkanes undergo free radical substitution reactions with halogens in presence of sunlight. A lot of products are going to form in minor amounts (less stable) and form a major product which is a highly stable one.
Complete step by step answer:
- In the Question it is given that 2-methyl butane reacts with chlorine in presence of sunlight and forms N isomers. And on fractional distillation it forms n distilled products.
- We have to identify the N and n.
- The chemical reaction of 2-methyl butane with Chlorine in presence of sunlight is as follows.
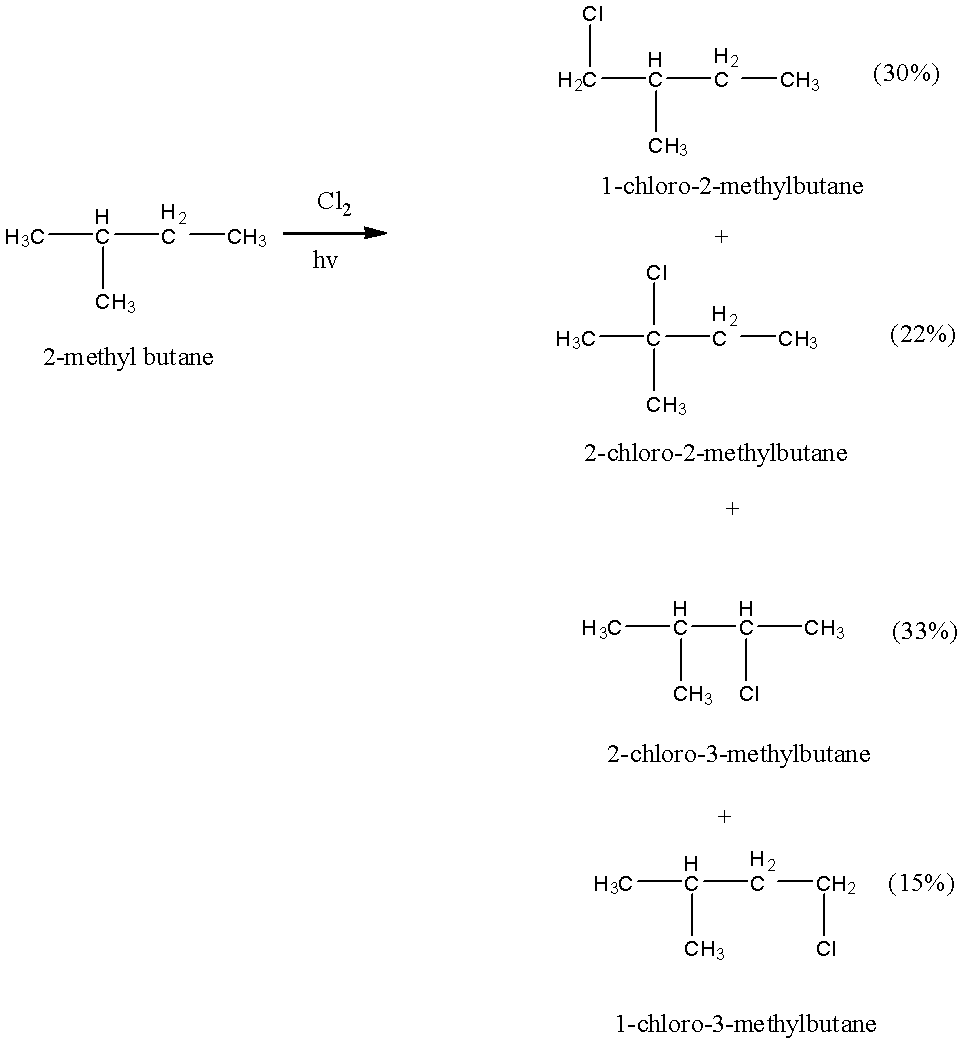
- 2-Methyl butane produces four different products with different percentages.
- The products formed are 22 % 2-chloro-2-methylbutane, 33 % 2-chloro-3-methylbutane, 30 % 1-chloro-2-methylbutane and 15 % 1-chloro-3-methylbutane.
- That means the value of N is four.
- The four products should have different boiling points due to the position of the chlorine being different from one another.
- Means on fractional distillation we will get four distilled products.
- Therefore N = 4 and n = 4.
So, the correct answer is “Option D”.
Note: By using fractional distillation we can separate the compounds on the basis of their boiling point. If a compound has less boiling point then it comes first and the compound which has a higher boiling point will come at last in the fractional distillation process.
