Question
Question: In the following reaction, \({C_2}{H_2}\xrightarrow[{HgS{O_4}/{H_2}S{O_4}}]{{{H_2}O}}X \rightlefth...
In the following reaction,
C2H2H2OHgSO4/H2SO4X⇌CH3CHO
What is X ?
(a) CH3CH2OH
(b) H3−O−CH3
(c) CH3CH2CHO
(d) CH2=CHOH
Solution
The reactant alkyne reacts with H2O in presence of HgSO4/H2SO4 and forms a product. So, we have to think about the reaction in which alkyne reacts with H2O in presence of HgSO4/H2SO4 and then tautomerism because the compound ( ⇌ this sign shows that this compound is tautomerising itself) is changing itself.
Complete answer:
So, we have to start with the reaction in which alkyne reacts with H2O in presence of HgSO4/H2SO4. So, that reaction is Alkyne Hydration.
Alkyne hydration:
So, we try to learn these reaction with an example,
So, let’s take an example,
CH3−C≡C−H+H2O→CH3COCH3
Mechanism:
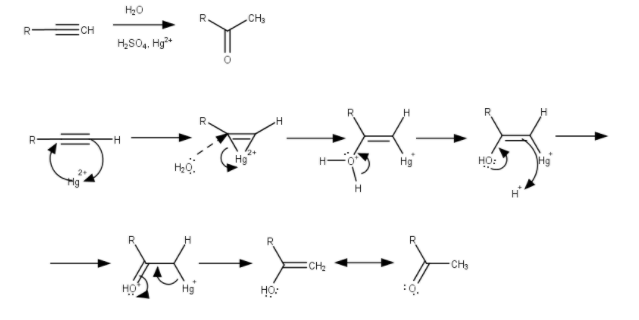
So, in case of unsymmetrical alkynes, the addition of water in the alkyne in according to the Markovnikov’s Rule, which states that the O− atom will attach with the carbon having less number of H− atoms and the two H− atoms will attach with the carbon having greater number of H− atoms.
But In these cases, the alkyne is symmetric.
So, we don’t have to follow any Markovnikov’s Rule,
And the reaction will be,
H−C≡C−HH2OHgSO4/H2SO4CH3CHO
Now, the CH3CHO will tautomerise itself,
So, let’s talk about tautomerism,
Tautomerism: When an aldehyde having one hydrogen atom, adjacent to the carbonyl group (i.e, α carbon), this hydrogen can move to the oxygen atom of the carbonyl group and the double bond between the carbonyl group is shifted to the adjacent to the carbonyl group (i.e, α carbon). This type of movement of bonds is called tautomerism and the compounds are called tautomers.
So, CH3CHO will tautomerise itself as
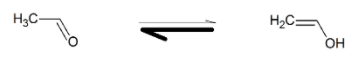
Hence, the complete reaction will be
C2H2H2OHgSO4/H2SO4CH2=CHOH⇌CH3CHO
Hence, the correct option is (D) LiF.
Note: While alkyne is reacting with H2O in presence of HgSO4/H2SO4 , we have to use the Markovnikov’s Rule when the alkyne is un-symmetric and when α carbon is present, we must have to think once about the tautomerism.
