Question
Question: In the following reaction, B is: \({{C}_{6}}{{H}_{5}}N{{H}_{2}}\xrightarrow{Bro\min ation}B\xright...
In the following reaction, B is:
C6H5NH2BrominationBNaNO2/HClCC2H5OHBoiling
(A) Salicylic acid
(B) Benzoic acid
(C) Phenol
(D) 2, 4, 6-Tribromoaniline
Solution
As we need to find the product B after bromination of a compound, the product will have bromine substitute in it.
Complete step by step solution:
Let us see when the aniline goes for bromination what happens.
The NH2 group in the aniline is a highly activating group. The lone pair of electrons present on the N releases the electron density to the benzene ring. This activates the benzene ring towards electrophilic substitution reactions at ortho and para positions.
In bromination Br+ is the electrophile. The multiple substitution takes place around the ring in all the activated positions.
1. Ortho- Structure has high electron density at ortho position. So, electrophile can attack the ortho position.
2. Para- Structure has high electron density at para position also. So, electrophile can attack the para position too.
Thus, when bromine water is added to aniline (also known as phenylamine), the bromine water is decolourised and the white precipitate is formed. In short, bromination of aniline takes place resulting in the multiple substitutions of an electrophile at activated positions i.e. ortho and para.
Thus, the end product will be 2, 4, 6-Tribromoaniline.
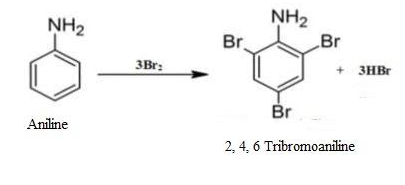
Therefore, option (D) is correct.
Note: Do note that the option (A), (B) and (C) can never be the answer factually as, bromination of any compound won’t result in formation of all those products given in the respective options i.e. salicylic acid, benzoic acid and phenol.
