Question
Question: In the following mono-bromination reaction, the number of possible chiral product(s) is (are)... ...
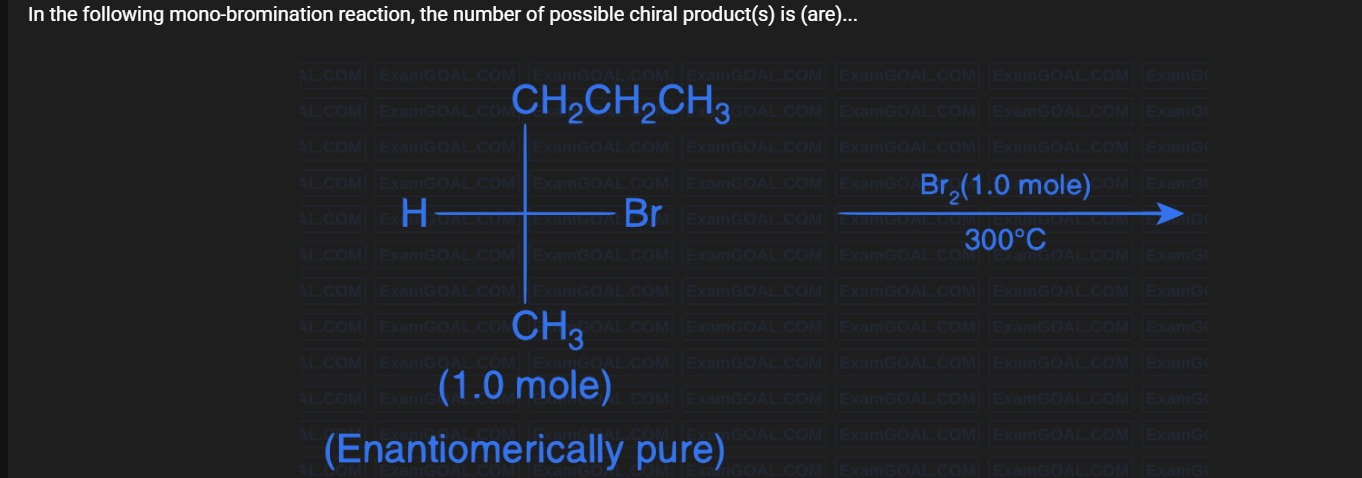
In the following mono-bromination reaction, the number of possible chiral product(s) is (are)...
Answer
6
Explanation
Solution
We start with a chiral molecule at a tetrahedral carbon (C*) with four different groups: H, Br, CH₃, and CH₂CH₂CH₃. In free‐radical bromination at 300°C, Br• can abstract any hydrogen. Let the sites be:
-
At C (the chiral center):*
- Replacing H gives C*(Br, Br, CH₃, CH₂CH₂CH₃) which is not chiral (two Br are identical).
-
On the CH₃ substituent:
- All 3 H’s are equivalent giving one product.
- The original chiral center remains so → product is chiral (1 product).
-
On the propyl group (CH₂CH₂CH₃):
(a) At the CH₂ directly attached to C (C₁'):*
- The two H’s are pro‐chiral; substitution creates a new stereocenter. Two configurations (diastereomers) are possible. → 2 chiral products.
(b) At the middle CH₂ (C₂'):
- Similarly, substitution gives a new chiral center yielding 2 diastereomers. → 2 chiral products.
(c) At the terminal CH₃:
- The three H’s are equivalent giving a CH₂Br group; no new stereocenter is formed, so only the original chiral center remains → 1 chiral product.
Adding these (excluding the achiral product from C*): 1 (from CH₃) + 2 (from C₁') + 2 (from C₂') + 1 (from terminal CH₃) = 6 chiral products.
Minimal Explanation:
- Bromination occurs at 4 different sites (ignoring substitution at C* which gives an achiral gem-dibromide).
- Substitution at the CH₃ and terminal CH₃ gives one product each.
- Substitution at each CH₂ of the propyl leads to a new stereocenter giving 2 products each.
Total = 1 + 2 + 2 + 1 = 6 chiral products.
