Question
Question: In the following compounds – (I) 
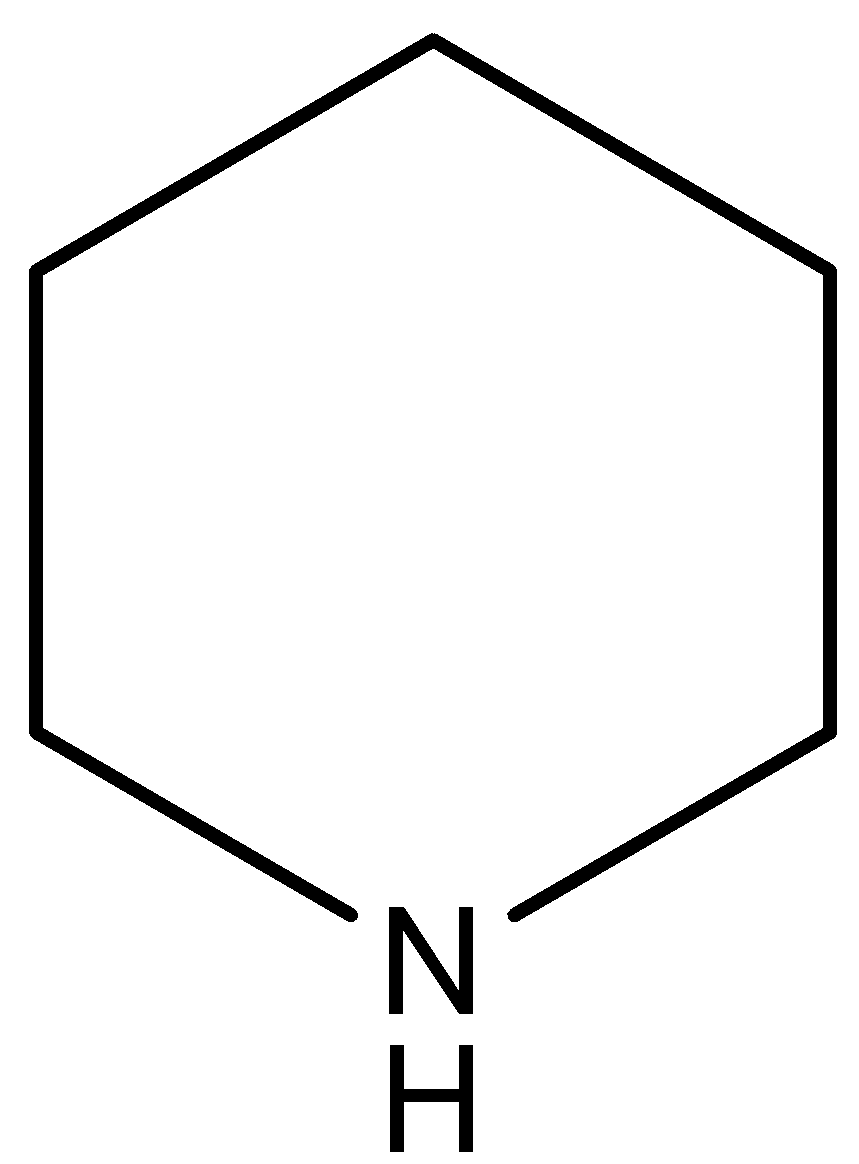
(II)
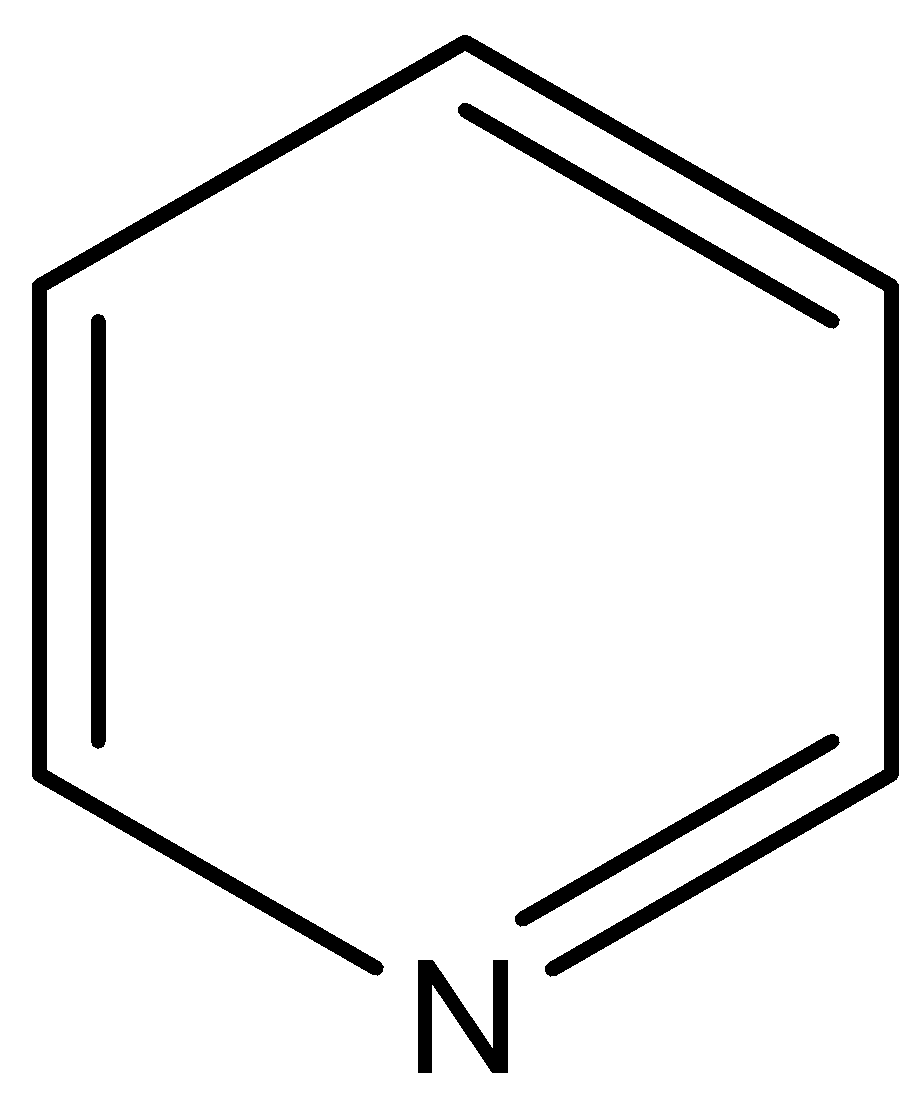
(III)
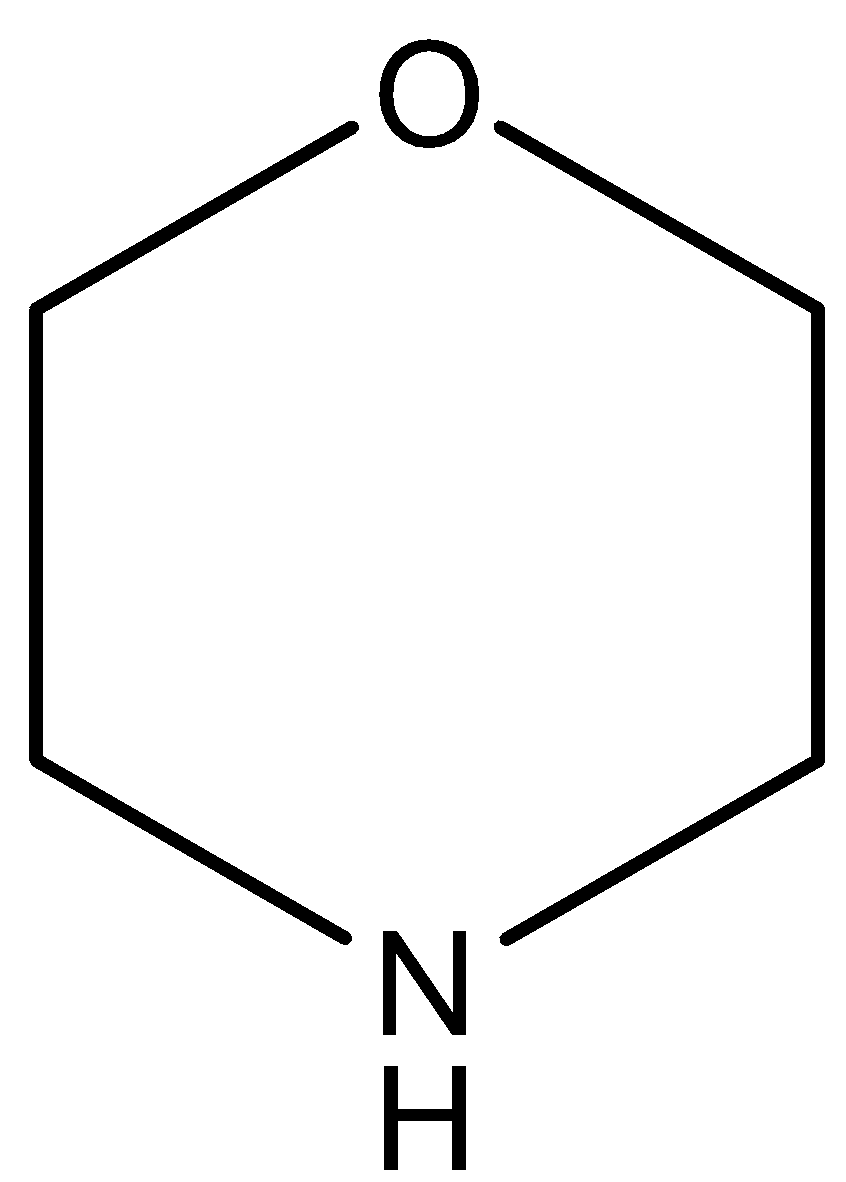
(IV)
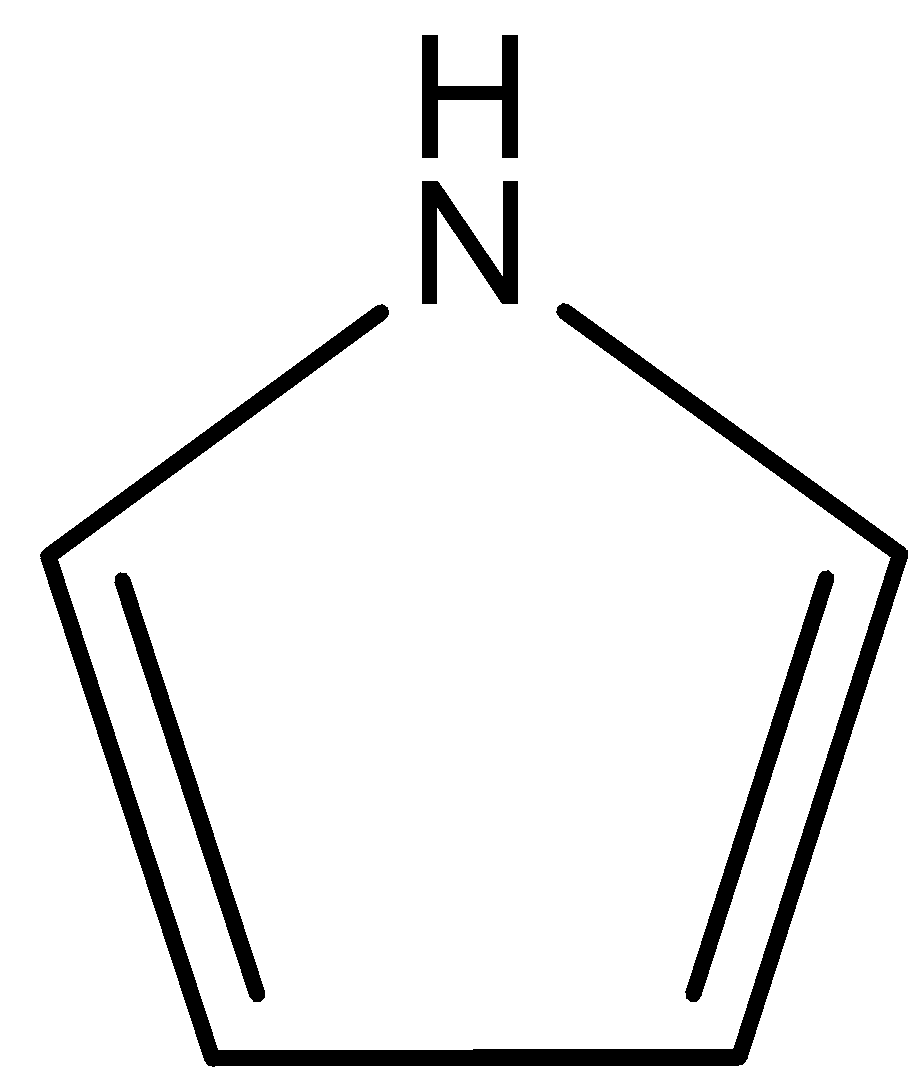
The order of basicity is
(A) IV>I>III>II
(B) III>I>IV>II
(C) II>I>III>IV
(D) I>III>II>IV
Solution
According to the Lewis concept electron pair acceptor compounds are acid, while electron pair donor compounds are base. Basicity of a base depends on the ease of electron donation by the donor compound, therefore compounds having a high tendency to donate electrons will be more basic.
Complete step by step answer:
The concept of organic chemistry that tells about basicity, acidity and also some of the parameters related to it are familiar to us from the lower classes.
Let us now understand this in detail by considering each compound given.
(A)
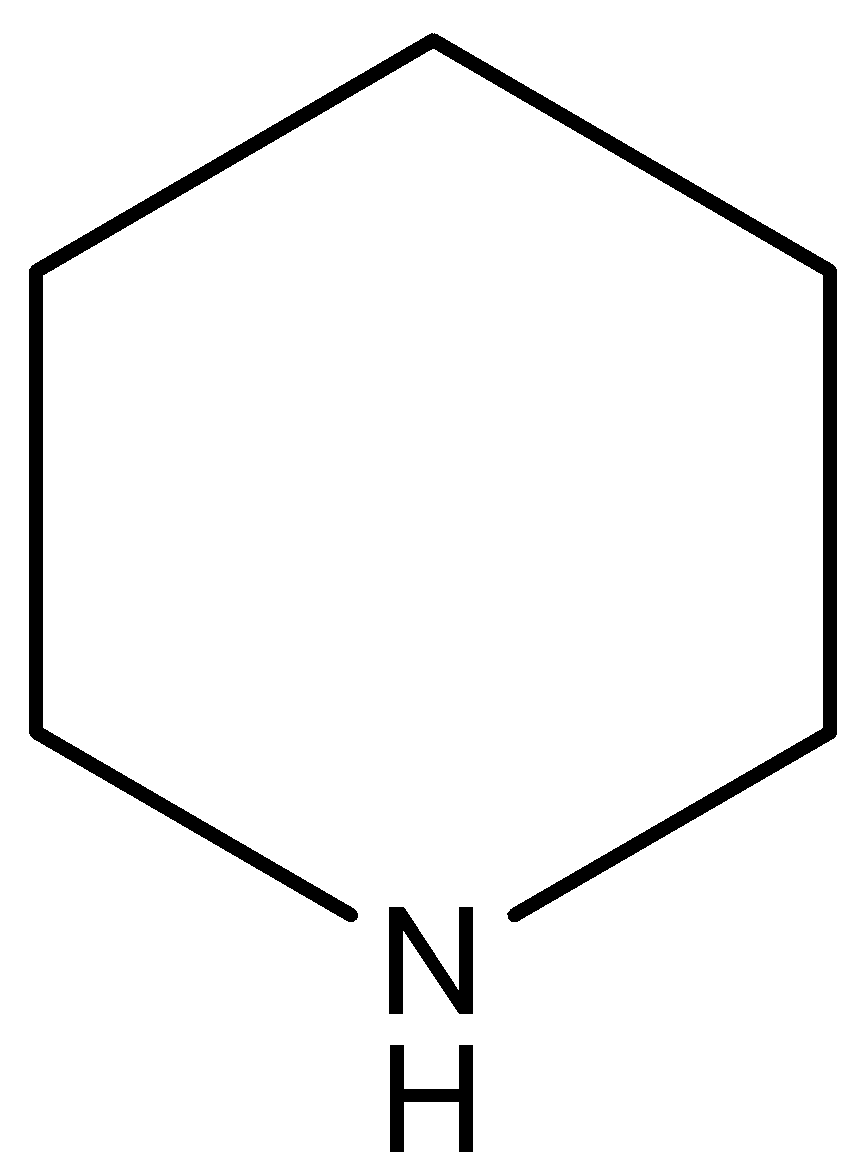
This compound is known as pyridine. This compound has the highest electron density on nitrogen atoms in respect to the other compounds. In this compound N-atom (electron pair donor) associated with two sp3 hybridized carbon atoms, so this compound has least inductive effect on the lone pair of the N-atom.
(B)
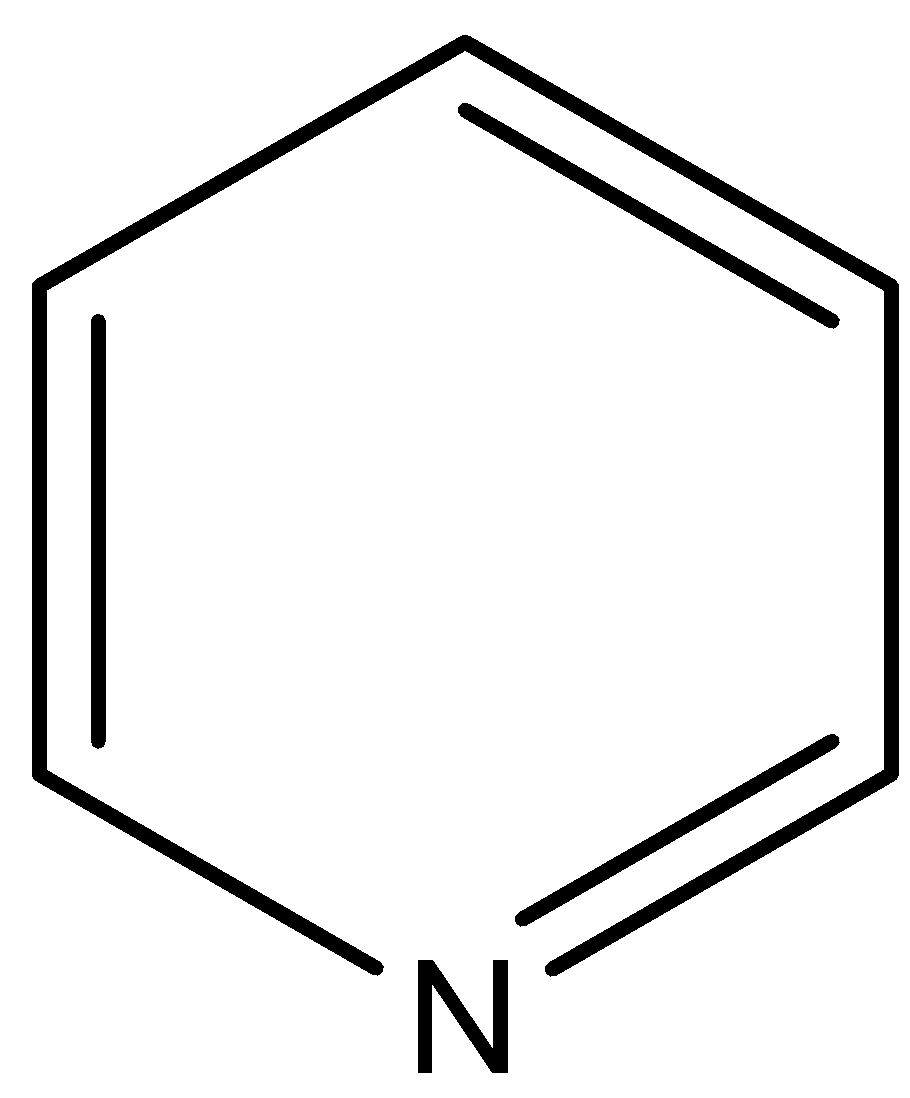
This compound is known as pyridine. The N atom of this compound is associated with two sp2 hybridized carbon atoms, so negative inductive effect of these sp2 hybridized carbon atoms decreases the electron density on N atom in respect to the pyridine.
(C)
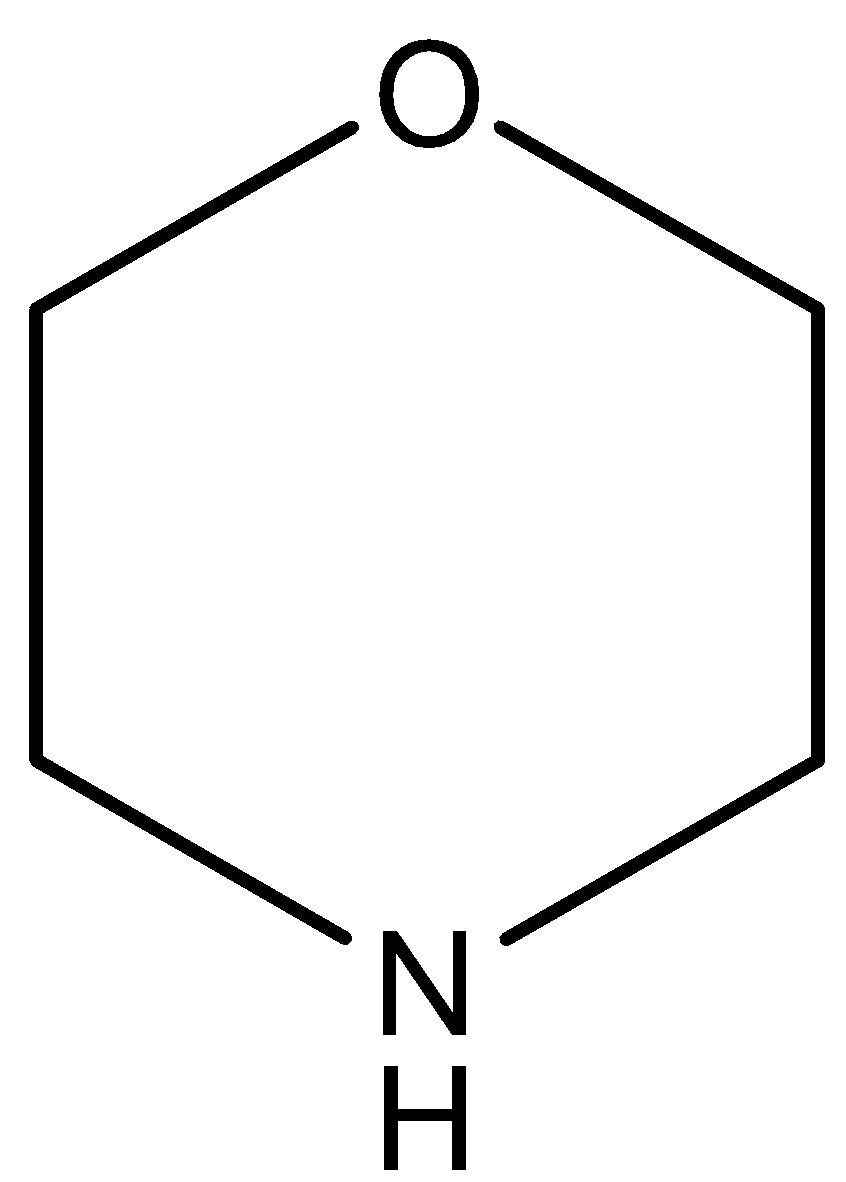
The oxygen atom of this compound has a negative inductive effect which decreases on increasing the distance from the N atom. So, the compound is more basic than pyridine and less basic than pyridine.
(D)
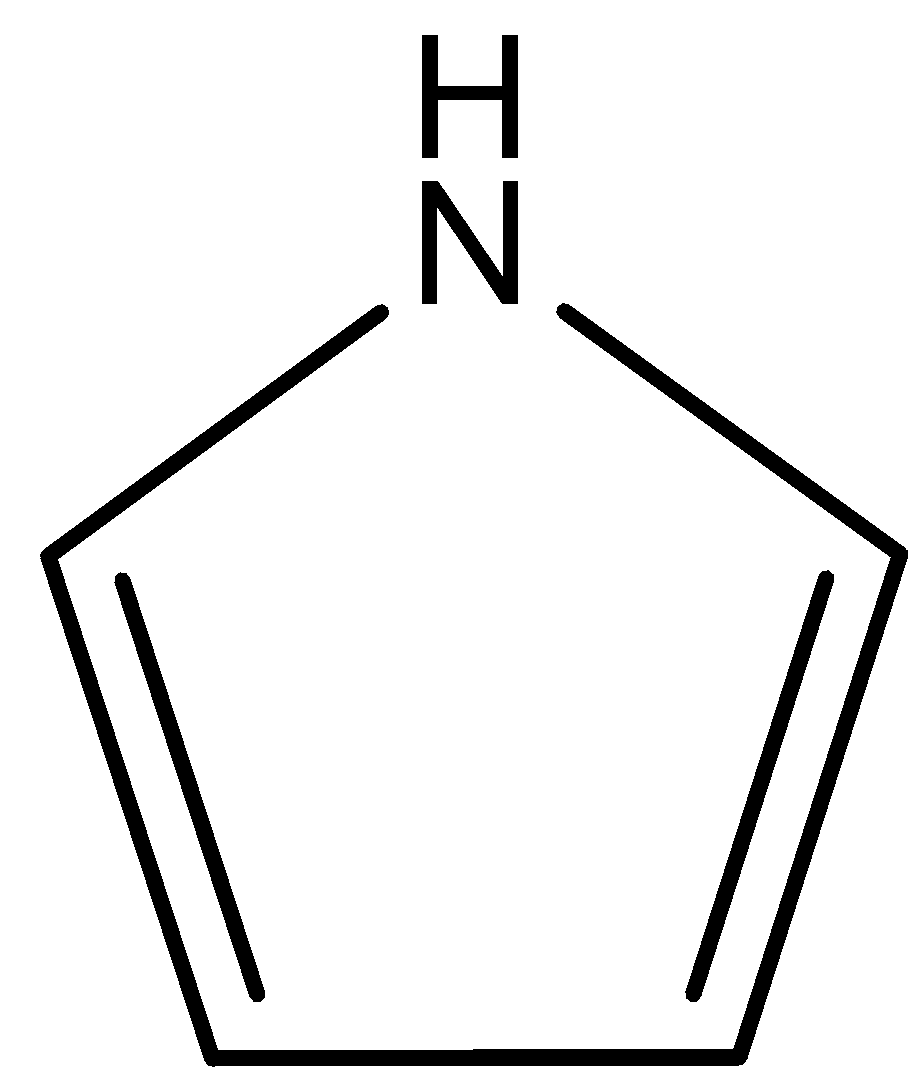
This compound is known as pyrrole. In this compound lone pair of N atoms is directly involved in the resonance with double bonds. So, lone pairs of N atoms are not available for donation. Therefore, this compound is least basic. The correct answer is option “D” .
Note: On increasing the s - character, the electronegativity of carbon atom increases. Those groups which have a negative inductive effect attract the shared pair of electrons toward itself and decrease the electron density on the donor atom.
