Question
Question: In the following compound, the number of sp hybridized carbons is: 
A. 2
B. 3
C. 4
D. 5
Solution
If a carbon is sp3 hybridized it contains four sigma bonds.
If a carbon is sp2 hybridized it contains three sigma bonds and one pi bond.
If a carbon is sp hybridized it contains two sigma bonds and two pi bonds.
Based on the above statements we can easily find the hybridization of carbons in a given molecule.
Complete step by step answer:
The structure of the given compound is
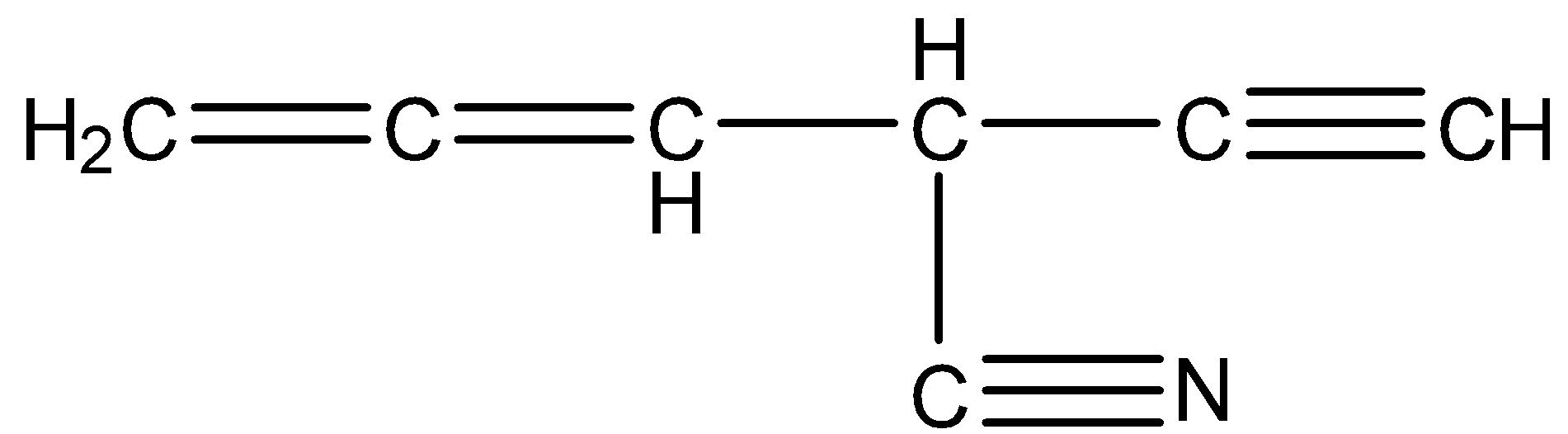
The number of carbons present in the given compound is 7.
Now we have to give numbers to all the carbons.
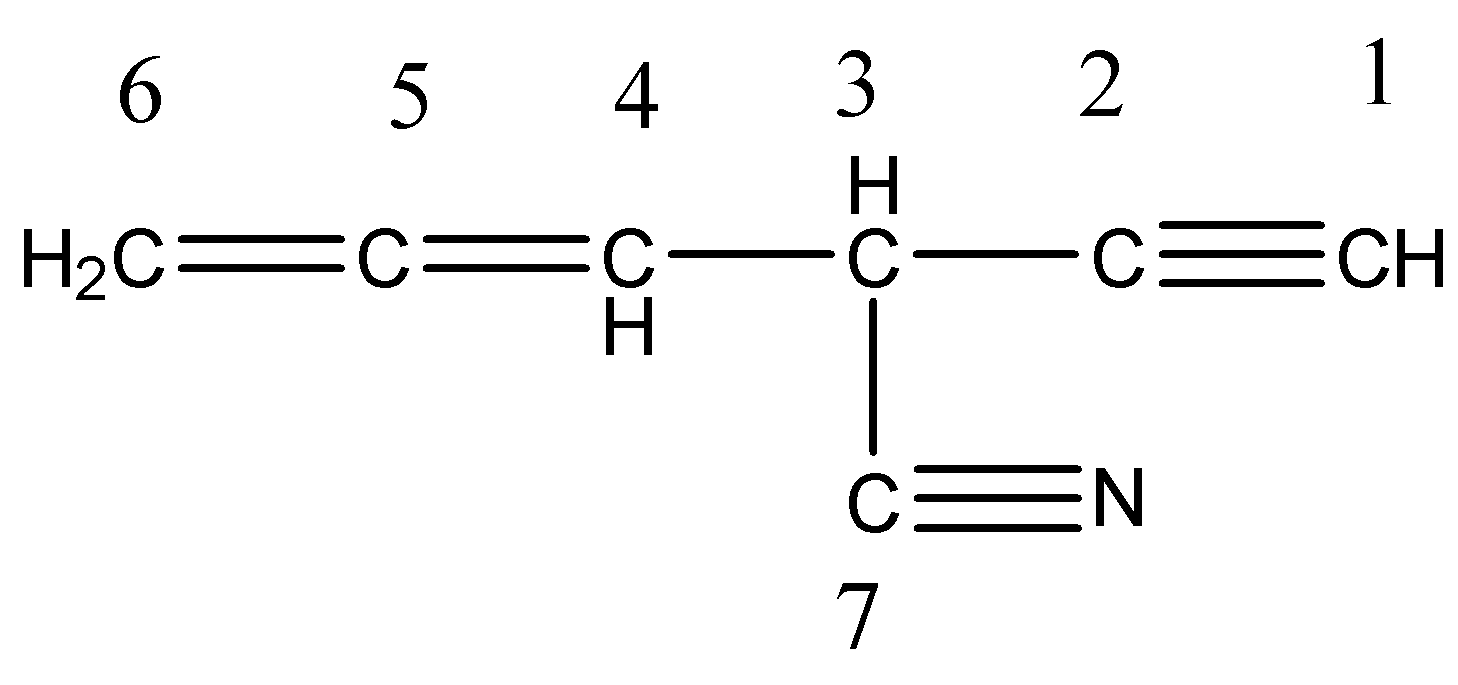
Coming to carbon number 1, it formed two sigma bonds (one with Hydrogen and the other with carbon-2) and two pi bonds (with carbon 2). So, carbon 1 is sp hybridized.
Coming to carbon number 2, it formed two sigma bonds (one sigma bond with carbon one and second sigma bond with carbon 3) and two pi bonds (with carbon 1). So, carbon 2 is sp hybridized.
Coming to carbon number 3, it formed four sigma bonds (one with carbon 2, second sigma bond with carbon 4, third sigma bond with carbon 7 and fourth sigma bond with hydrogen). So, carbon 3 is sp3 hybridized.
Coming to carbon number 4, it formed three sigma bonds (with carbon 3, with carbon 5 and with hydrogen) and one pi bond (with carbon 5). So, carbon 4 issp2 hybridized.
Coming to carbon 5, it formed two sigma bonds (with carob 4 and with carbon 6) and two pi bonds (with carbon 4 and with carbon 5). So, carbon 5 is sp hybridized.
Coming to carbon 6, it formed three sigma bonds (with carbon 5 and with two hydrogens) and one pi bond (with carbon 5). So, carbon 6 is sp2 hybridized.
Coming to carbon 7. It formed two sigma bonds (with carbon 3 and with nitrogen) and two pi bonds (with nitrogen). So, carbon 7 is sp hybridized.
Therefore there are four sp hybridized carbons (carbon number 1, 2, 5, and 7) in the given compound.
So, the correct option is C.
Note: In a double bond the type of bonds present are one sigma bond and one pi bond, in triple bond the types of bonds present are one sigma bond and two pi bonds. Sigma bond is going to form through axial overlapping of orbitals and pi bond is going to form through sidewise overlapping of orbitals.
