Question
Question: In the following acid-base reaction, how would you identify the acid, base and their conjugate acids...
In the following acid-base reaction, how would you identify the acid, base and their conjugate acids and bases:
NH4++HCO3−→NH3+H2CO3
Solution
When an acid and base get reacted it forms a salt. The parent acid and the parent base can be determined by splitting the salt into the cationic part and anionic part. The pH of salt will be neutral, that’s the reason when acid and base react the neutralization takes place. Since, the acid pH lies between pH1−6 and base pH lies between pH8−14 .
Complete step-by-step answer: An acid is a substance which can donate its proton. The base is a substance which can donate its hydroxy ions. Base and acid react to form salt and water. The hydroxy comes from base and proton from the acid to form water.
Salt can be defined as the neutralization of a product from acid and base.
Let’s discuss the Lewis acid and Lewis base;
Lewis acid: the ion which can accept the pair of electrons also known as Bronsted-lowry base.
Lewis base: the ion which can donate the pair of electrons also known as Bronsted-lowry acid.
The change fromNH4+ to NH3 shows that a proton was donated from NH4+, so simply put, NH4+is the acid, and we know thatNH3 is the base.
Since NH3 is the neutral state, NH4+is the conjugate acid.
The change from HCO3− to H2CO3 shows that a proton was accepted by HCO3−, so simply put, HCO3−is the base, and H2CO3 is the acid.
Since H2CO3 is the neutral state, HCO3−is the conjugate base.
Overall, we have:
NH4+:
Proton donor (Bronsted -Lowry acid)
Electron acceptor (Lewis acid)
NH3:
Proton acceptor (Bronsted -Lowry base)
Electron donor (Lewis base)
HCO3−:
Proton acceptor (Bronsted -Lowry base)
Electron donor (Lewis base)
H2CO3:
Proton donor (Bronsted -Lowry acid)
Electron acceptor (Lewis acid)
Let’s observe the diagram of how it shows;
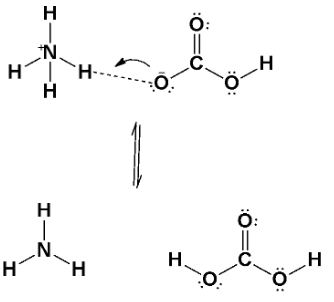
Note: A Lewis acid is a substance, such as the H+ ion, that can accept a pair of nonbonding electrons. In simple words, a Lewis acid is an electron-pair acceptor. A Lewis base is any substance, such as the OH− ion, that can donate a pair of nonbonding electrons. A Lewis base is, therefore, an electron-pair donor.
