Question
Question: In the figure shown, AB is a rod of length 30 cm and area of cross-section 1.0 $cm^2$ and thermal co...
In the figure shown, AB is a rod of length 30 cm and area of cross-section 1.0 cm2 and thermal conductivity 336 S.I. units. The ends A & B are maintained at temperatures 20° C and 40° C respectively. A point C of this rod is connected to a box D, containing ice at 0° C, through a highly conducting wire of negligible heat capacity. The rate at which ice melts in the box is : [Latent heat of fusion for ice L, = 80 cal/gm]
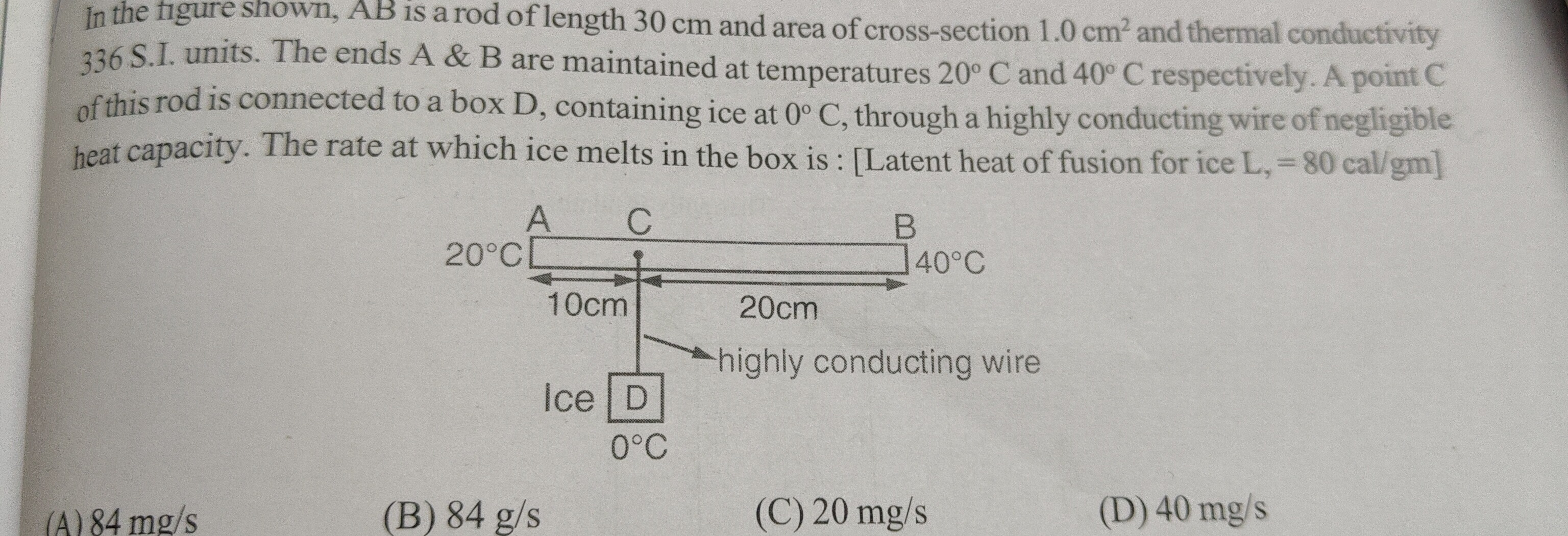
84 mg/s
84 g/s
20 mg/s
40 mg/s
40 mg/s
Solution
The problem involves heat conduction through a rod and the melting of ice. We are given a rod AB with ends maintained at temperatures TA=20∘C and TB=40∘C. Point C on the rod is connected to ice at TC=0∘C via a highly conducting wire, implying that the temperature at point C is TC=0∘C.
The rod has length LAB=30 cm, area of cross-section A=1.0cm2, and thermal conductivity k=336 S.I. units. Point C is located 10 cm from A (LAC=10 cm) and 20 cm from B (LCB=20 cm). The latent heat of fusion for ice is Lf=80 cal/gm.
Since TA>TC, heat flows from end A to point C. The rate of heat flow through section AC is given by Fourier's law: HAC=kALACTA−TC
Since TB>TC, heat flows from end B to point C. The rate of heat flow through section CB is: HCB=kALCBTB−TC
We need to convert all units to SI units for calculation. A=1.0cm2=1.0×10−4m2 LAC=10cm=0.1m LCB=20cm=0.2m k=336W/(m⋅K)
Now, calculate the heat flow rates: HAC=336W/(m⋅K)×(1.0×10−4m2)×0.1m20∘C−0∘C=336×10−4×0.120=336×10−4×200=6.72W
HCB=336W/(m⋅K)×(1.0×10−4m2)×0.2m40∘C−0∘C=336×10−4×0.240=336×10−4×200=6.72W
The total rate at which heat is transferred to point C from the rod is the sum of these two heat flows: Hmelt=HAC+HCB=6.72W+6.72W=13.44W
This heat is used to melt the ice. The rate of melting of ice (dtdm) is given by Hmelt=dtdm×Lf. We need to use consistent units for Lf. Given Lf=80 cal/gm. We use the conversion factor 1 cal = 4.184 J. Lf=80gmcal=80×10−3kg4.184J=80×4184J/kg=334720J/kg.
Now, calculate the rate of mass melting: dtdm=LfHmelt=334720J/kg13.44J/s≈4.015296×10−5kg/s
The options are in mg/s. Convert kg/s to mg/s: 1kg=106mg. dtdm≈4.015296×10−5kg/s×106mg/kg=40.15296mg/s.
Rounding to the nearest option, the rate at which ice melts is approximately 40 mg/s.
