Question
Question: In the extraction of aluminum metal, one of the process is summarized as follows: Which of the fol...
In the extraction of aluminum metal, one of the process is summarized as follows:
Which of the following entries summarizes reagents, electrodes and products of the process?
In the extraction of aluminum metal, one of the process is summarized as follows:
Which of the following entries summarizes reagents, electrodes and products of the process?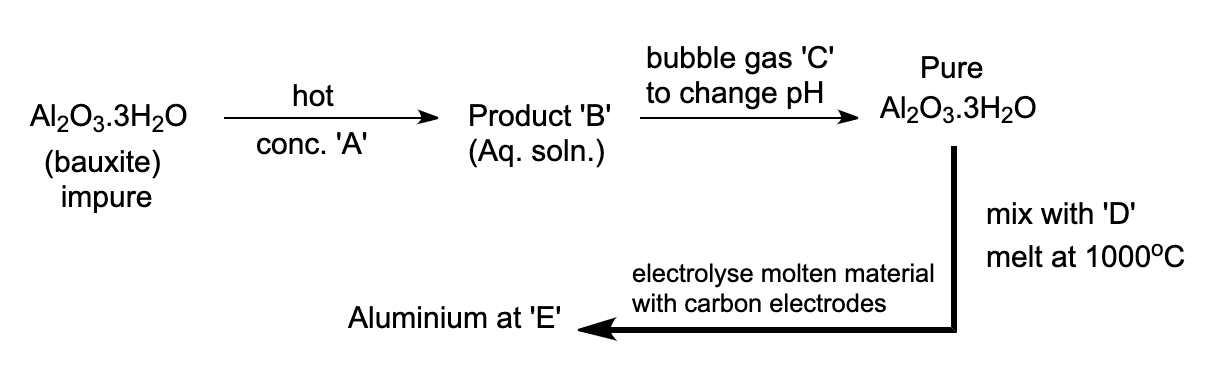
(a)- NaOHA Al3+B HFC Na3AlF6D CathodeF
(b)- NaOHA NaAlO2B CO2C NaFD AnodeF
(c)- H2SO4A Al2(SO4)3B NH3C Na3AlF6D CathodeF
(d)- NaOHA NaAlO2B CO2C Na3AlF6D CathodeF
Solution
Bayer’s process is a method in which we can extract the aluminium and then the electrolysis is done. In Bayer’s process we obtain aluminium oxide, a white powder from which aluminium can be extracted.
Complete answer:
- Aluminium is mainly extracted from the bauxite ore. Bauxite is an important ore of aluminium with the molecular formula Al2O3.3H2O
- In the process of concentration of bauxite, the ore of aluminium i.e., bauxite, is heated in a pressure vessel along with a concentrated solution of sodium hydroxide also known as caustic soda at high temperature. The aluminium will be dissolved as aluminate ion at this temperature and it can be further processed for extraction. At cathode, aluminium starts to deposit, this is pure aluminium.
- In the Hall-Heroult’s process, carbon dioxide is bubbled in order to change the pH of the reaction and to obtain pure aluminium. Later, this pure aluminium ore is mixed with cryolite Na3AlF6. This process is done in a graphite rod and a steel vessel that is lined with carbon.
- Therefore, according to the above statements the correct option is (D).
Note:
The extraction is done with the help of an electrolytic process which is named as Hall-Heroult’s process, and the ions in the aluminium oxide should be free because by free electrons only the current will be passed easily.
