Question
Question: In the electrolysis of aqueous $CuBr_2$, using Pt electrodes, what are the products obtained at anod...
In the electrolysis of aqueous CuBr2, using Pt electrodes, what are the products obtained at anode and cathode?
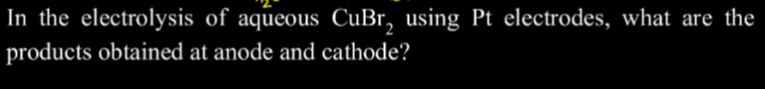
Answer
Cu at cathode, Br2 at anode
Explanation
Solution
Species in aqueous CuBr2: Cu2+, Br−, H2O. Pt electrodes are inert.
At cathode (reduction): Compare reduction potentials of Cu2+ (+0.34V) and H2O (−0.83V). Cu2+ is reduced as it has higher reduction potential. Cathode product: Cu.
At anode (oxidation): Compare reduction potentials of Br2/Br− (+1.09V) and O2/H2O (+1.23V). Species with lower reduction potential is oxidized. Br− is oxidized as 1.09V<1.23V. Anode product: Br2.
Answer: Cu at cathode, Br2 at anode.
