Question
Question: In the diagram shown \[{{\text{Q}}_{{\text{iaf}}}} = 80\;{\text{cal}}\] and \[{{\text{W}}_{{\text{ia...
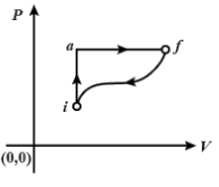
In the diagram shown Qiaf=80cal and Wiaf=50cal. If W=−30cal for the curved path fi, the value of Q for the path fi , will be:
A.60 cal
B.30 cal
C.−30cal
D.−60cal
Solution
To answer this question, you should recall the concept of the first law of thermodynamics. According to this law, the change in internal energy of a system depends on the net heat transfer into the system and the network done by the system.
The formula used:
ΔU = ΔQ − ΔW
where ΔU is the change in internal energy of the system, ΔQ is heat transfer and ΔWis work done on the system
Complete step by step answer:
The equation of the first law of thermodynamics is
ΔU = ΔQ − ΔW.
The values of the conditions along the path iafas
Qiaf=80 cal, Wiaf=50 cal.
The question requires us to find us the value of conditions for the path fi, Wfi=−30 cal,Qfi=?.
Along the path fi,ΔU=Q−W=80−50=30cal
ΔUfor fi=−30cal
Using these values in the equation of first law we get
Q=W+ΔU=−30−30=−60cal.
Q for path fi= −60cal.
Hence, the final answer is D.
Note:
We can confuse between different types of reactions. Make sure to remember the difference between isobaric, isochoric, isothermal and adiabatic processes. An isobaric process is one where the pressure of the system (often a gas) stays constant.
An isochoric process is defined as the thermodynamic process where the volume of the system stays constant.
An adiabatic process is a thermodynamic process in which no heat is exchanged between the system and the surrounding. The limitation of this first law of thermodynamics is that it fails to explain why heat flows from hot end to cold end when a metallic rod is heated at one end, not in another case and vice-versa. This means that the first law only quantifies the energy transfer that takes place during this process. It is the second law of thermodynamics which provides the criterion for the feasibility of the various processes.
