Question
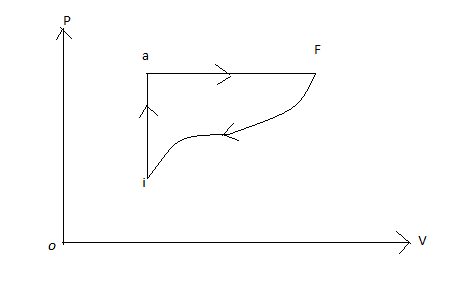
Question: In the diagram shown \({Q_{iaf}} = 80cal\) and \({W_{iaf}} = 50cal\) . If \(W = ( - 30)cal\) for the...
In the diagram shown Qiaf=80cal and Wiaf=50cal . If W=(−30)cal for the curved path fi, find the Q for path fi?
a. 60cal
b. 30cal
c. −30cal
d. −60cal
Solution
Hint In the entire solution only one formula will be used repeatedly that is
Q=ΔU+W where Q is the heat, U is the internal energy and W is the work done.
We will also have to take care of sign conventions . We will also make use of the fact that U(internal energy) is a state function, that means it does not depend upon the path followed but only the initial and final states.
Complete step-by-step solution :
First of all, we will consider path iaf. We are given Qiaf=80cal and Wiaf=50cal
The change in internal energy can then be calculated by using the first law of thermodynamics, which states that
Qiaf=ΔU+Wiaf
Since , ΔU only depends on initial and final states , here ΔU=Uf−Ui
Now, substituting the values in the equation we will get
80 = \Delta U + {W_{iaf}} \\\
80 = \Delta U + 50 \\\
\Delta U = 80 - 50 \\\
\Delta U = 30cal \\\
{U_f} - {U_i} = 30cal \\\
Now, we will move from f to i through the curved path .
We are given that work done in moving f to i is
Wfi=(−30cal)
The change in internal energy while moving from f to i will be
ΔU=Uf−Ui=(−(Ui−Uf))=(−30cal)
We shall again make the use of the relation we had used before for path fi,
Qfi=ΔU+Wfi
Substituting the values, we have
{Q_{fi}} = - 30 - 30 \\\
{Q_{fi}} = - 60cal \\\
So, option (d.) is correct
Note:-
In this type of problem we need to take care of the directions in which we are moving. While moving through the same path but in the opposite direction, we need to use the opposite sign. So, good care must be taken in the use of signs.
