Question
Question: In the diagram, how are valence electrons illustrated? How many valence electrons does each element ...
In the diagram, how are valence electrons illustrated? How many valence electrons does each element have?
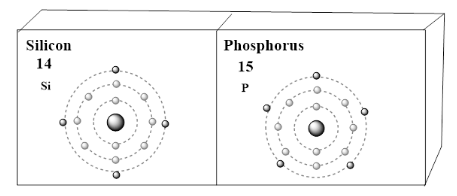
Solution
Valence electrons are electrons in an atom's external energy level that can participate in interactions with other atoms. The valence electrons are usually the most distant electrons from the nucleus. As a result, they may be attracted as much or more to the nucleus of another atom than to their nucleus.
Complete answer:
The electronic configuration says about how the electrons are distributed in atomic orbitals. The electronic configuration is filled according to the energy they have. They follow the standard notion in which electrons containing the atomic subshells are placed in a sequence. For example, the Magnesium’s electron configuration; Mg= 1s22s22p63s2
The full electronic configuration of any element is written in a standard format. But when it comes to higher elements will consist of many numbers of orbital, so for simplification condensed electronic configuration is noted.

The full electronic configuration of silicon;
Si= 1s22s22p63s23p2
The full electronic configuration of phosphorus;
P= 1s22s22p63s23p3
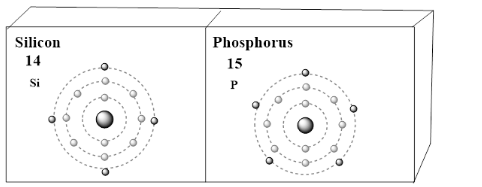
In the case of valence electrons of silicon and phosphorus the outermost electrons present in the darker shade is the valence. These valence electrons are crucial to effective chemical bonding and are usually the sole participants in chemical reactions (as opposed to core participation).
Thus, there are 4 valence electrons in silicon atoms and 5 in phosphorus atoms.
Note: Silicon (Si) whose atomic number is 14 , which is having 14 electrons. The 14 protons are there in atomic nuclei. A neutral atom has the same number of protons as well as the same number of electrons. When it comes to the case of silicon, it is in the 3rd period of the table and the group 14 element. The silicon has 4 valence electrons. Phosphorus (P) whose atomic number is 15 , which is having 15 electrons. The 15 protons are there in atomic nuclei. A neutral atom has the same number of protons as well as the same number of electrons. When it comes to the case of phosphorus, it is in the 3rd period of the table and the group 15 element. The phosphorus has 5 valence electrons.
