Question
Question: In the conversion of alkyne to trans-alkene by Birch reduction using alkali metals (such as Na or K)...
In the conversion of alkyne to trans-alkene by Birch reduction using alkali metals (such as Na or K) in liquid NH3 and alcohol (MeOH or EtOH). What is the mechanism taking place in the formation of intermediate species in the following sequence
R−≡−RNa+liq.NH3+EtOHHR⟩=⟨RH
(A) Radical anion → vinylic radical → trans-vinylic anion → trans alkene
(B) Radical anion → trans-vinylic anion → vinylic radical → trans alkene
(C) Vinylic radical → Radical anion → trans-vinylic anion → trans alkene
(D) Vinylic radical → trans-vinylic anion → radical anion → trans alkene
Solution
The conversion of alkyne to trans-alkene by Birch reduction using alkali metals (such as Na or K) in liquid NH3 and alcohol (MeOH or EtOH), four radicals are formed i.e. radical anion, vinylic radical, trans-vinylic anion, trans alkene.
Complete step by step solution:
Let us first discuss the Birch reduction-
-The Birch reduction is an organic chemical reaction where aromatic compounds which have a benzenoid ring are converted into 1,4-cyclohexadiene which have two hydrogen atoms attached opposite ends of the molecule. It is a very useful reaction in synthetic organic chemistry.
-Conjugated enamines can also be formed from the Birch reduction of aniline. Alkynes can also undergo Birch reduction to form alkenes.
-Birch reduction is named after the Australian chemist Arthur Birch. The reaction type involved in this process is organic redox reaction.
-Alkynes are selectively converted into trans alkenes when they are reduced by a solution of sodium (or lithium) in liquid that contains stoichiometric amounts of an alcohol such as ethanol.
-The first step of this reduction is one-electron transfer into an antibonding π orbital of alkyne, which yields a radical anion.
-Subsequently, protonation of the radical anion, an additional one-electron transfer, and a concluding protonation yield a trans alkene.
Illustration- The mechanism:
-Reduction of the alkyne by sodium results in breakage of the C-C double bond and formation of an anion adjacent to the radical.
-The radical that is formed can interconvert between its cis and trans form, but the trans is generally more stable due to steric factors. The anion is then protonated by NH3 (the only acid present in solution) to give the vinyl radical, which is then reduced by a second equivalent of Na to give a second anion. This is then converted to the alkene by protonation with the second equivalent of NH3. So, the net process gives a trans alkene and the two equivalents of NaNH2.
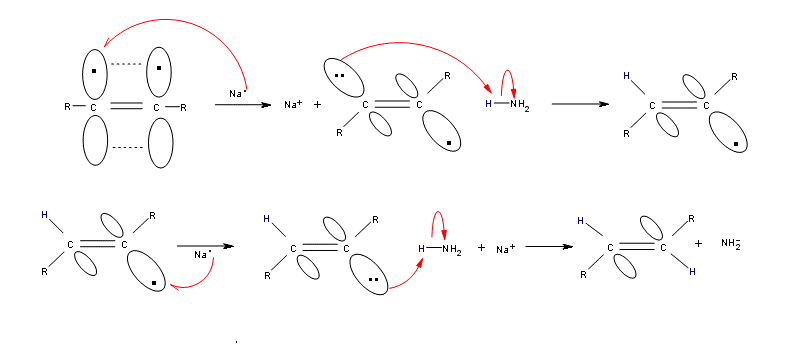
Therefore, option (A) Radical anion → vinylic radical → trans-vinylic anion → trans alkene is correct.
Note: Do not get confused between vinylic radical and vinylic anion. Always remember that the radical is formed prior to the anion formation.
