Question
Question: In the compound \[{\text{CoC}}{{\text{l}}_{\text{3}}}{\text{.5N}}{{\text{H}}_{\text{3}}}\] : (A) ...
In the compound CoCl3.5NH3 :
(A) all the chlorine shows primary valency only.
(B) Two chlorines show primary valency and one chlorine shows secondary valency.
(C) Two chlorines show primary valency and one chlorine shows primary valency as well as secondary valency.
(D) All then show secondary valency.
Solution
Two spheres, coordination sphere and ionization sphere are present around the central metal atom. Primary valence represents the ionization sphere and the secondary valence represents the coordination sphere.
Complete answer:
The compound CoCl3.5NH3 is a coordination compound. It contains 5 ammonia ligands directly bonded to central cobalt metal. The coordination number of cobalt is 6. So central cobalt metal can have only one other ligand. This another ligand is in the form of one chlorine atom. Thus, the coordination sphere of central cobalt metal contains five ammonia ligands and one chlorine ligand. The remaining two chlorine ligands are present in the ionization sphere.
The ligands present in the coordination sphere are satisfying the secondary valence. Thus, five ammonia molecules and one chlorine ligand are satisfying the secondary valence of cobalt and are present in the coordination sphere. The remaining two chlorine atoms are present in the ionization sphere and satisfy the primary valence (or the oxidation state) of cobalt metal.
The formula of the coordination compound can be written as [CoCl(NH3)5]⋅Cl2 .
Thus, in the compound CoCl3.5NH3, two chlorine show primary valency and one chlorine shows primary valency as well as secondary valency.
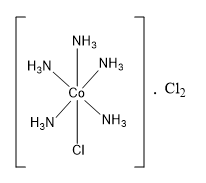
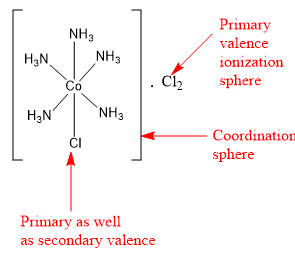
Hence, the correct option is the option (C).
Note: There are some examples, in which the same ligand satisfies primary as well as secondary valence. In the coordination compound [CoCl(NH3)5]⋅Cl2 the chlorine ligand present inside the square brackets, satisfies both primary valence and the secondary valence.
