Question
Question: In the complexes, \({\left[ {Fe{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }},{\left[ {Fe{{\left( {C...
In the complexes, [Fe(H2O)6]3+,[Fe(CN)6]3−,[Fe(C2O4)3]3−and [FeCl6]3−, more stability is shown by:
A. [FeCl6]3−
B. [Fe(C2O4)3]3−
C. [Fe(H2O)6]3+
D. [Fe(CN)6]3−
Solution
The stability in a complex can be determined by considering the chelates present in the complex. Chelate refers to a ligand which is bonded to the central metal atom at two or more than two points.
Complete step by step answer:
We have to draw the structure of the complexes to determine which has more stability. Following are the structures of the four given complexes.
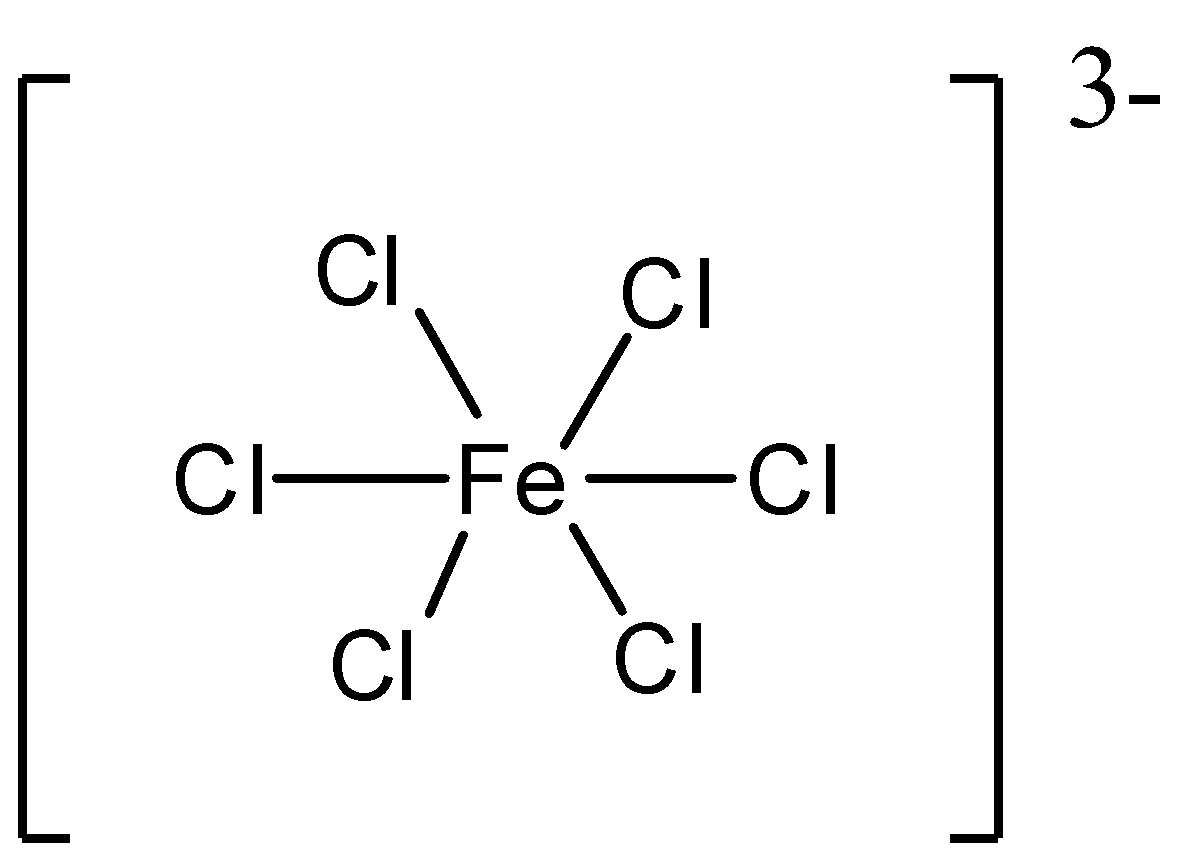
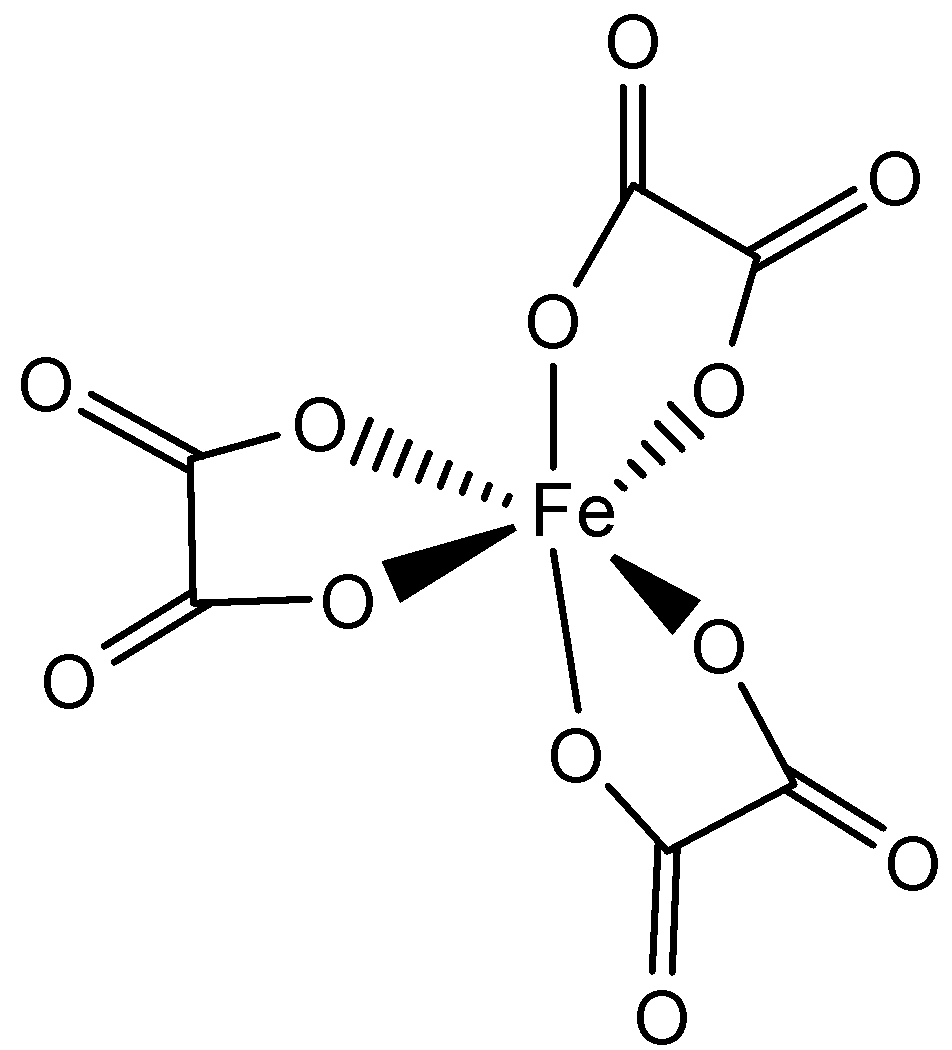
A. B..
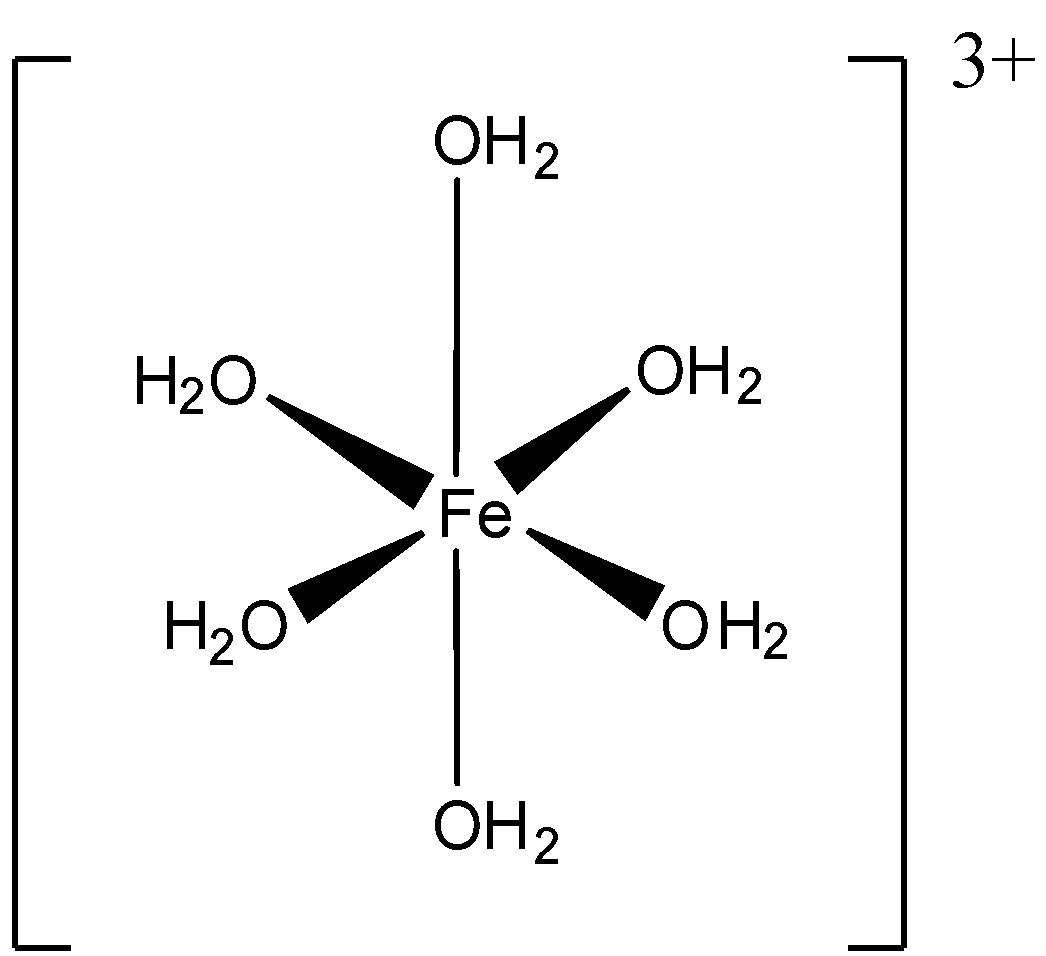
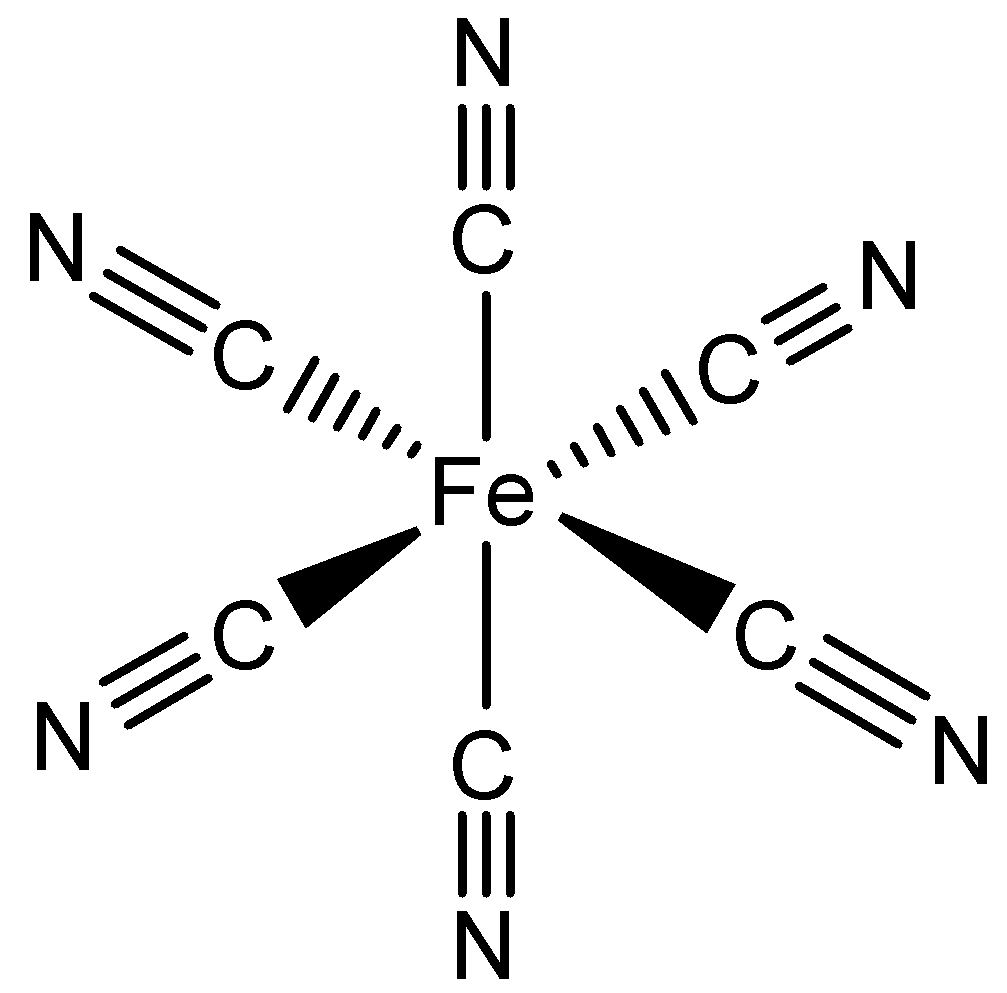
C. D.
From the above structures, we see that [Fe(C2O4)3]3−consists of maximum number of chelates. In [Fe(C2O4)3]3−, Fe is in +3 oxidation state and as the C2O42− is a bidentate chelating ligand, it forms rings. We know that with the increase in the number of chelates present, the stability of complexes increases. So, the complex [Fe(C2O4)3]3−is the most stable out of the four.
Therefore B is the correct option.
Additional information:
Chelating ligands are ligands that can attach to the metal atoms in two or more than two points. The compounds made by these chelating ligands are called chelates. A chelating ring may have any number of atoms. Chelating ligands are ligands that form several bonds to one single metal ion. They are called multidentate ligands. Some examples of chelating ligands are acetylacetone, EDTA and ethylenediamine.
Note:
In a complex, the stability of a complex is determined by the number of chelates. The stability of a complex increases with the increase in the number of chelates.
