Question
Question: In the complex acetyl bromide dicarbonyl bis (triethylphosphine) iron (II), the number of \(Fe-C\) b...
In the complex acetyl bromide dicarbonyl bis (triethylphosphine) iron (II), the number of Fe−C bond is:
Solution
Think about the ligands which are present in the complex. Count the number of ligands and then check the type of ligands present. Write the molecular formula of each ligand and then identify the atom in the ligand forming bond with the central metal atom that is, iron. Count the total number of metal-carbon bonds formed to get the answer.
Complete answer:
- In the complex acetyl bromide dicarbonyl bis (triethylphosphine) iron (II), iron is in +2 oxidation state.
We shall see the structure of the complex and then calculate the number ofFe−Cbonds in it.
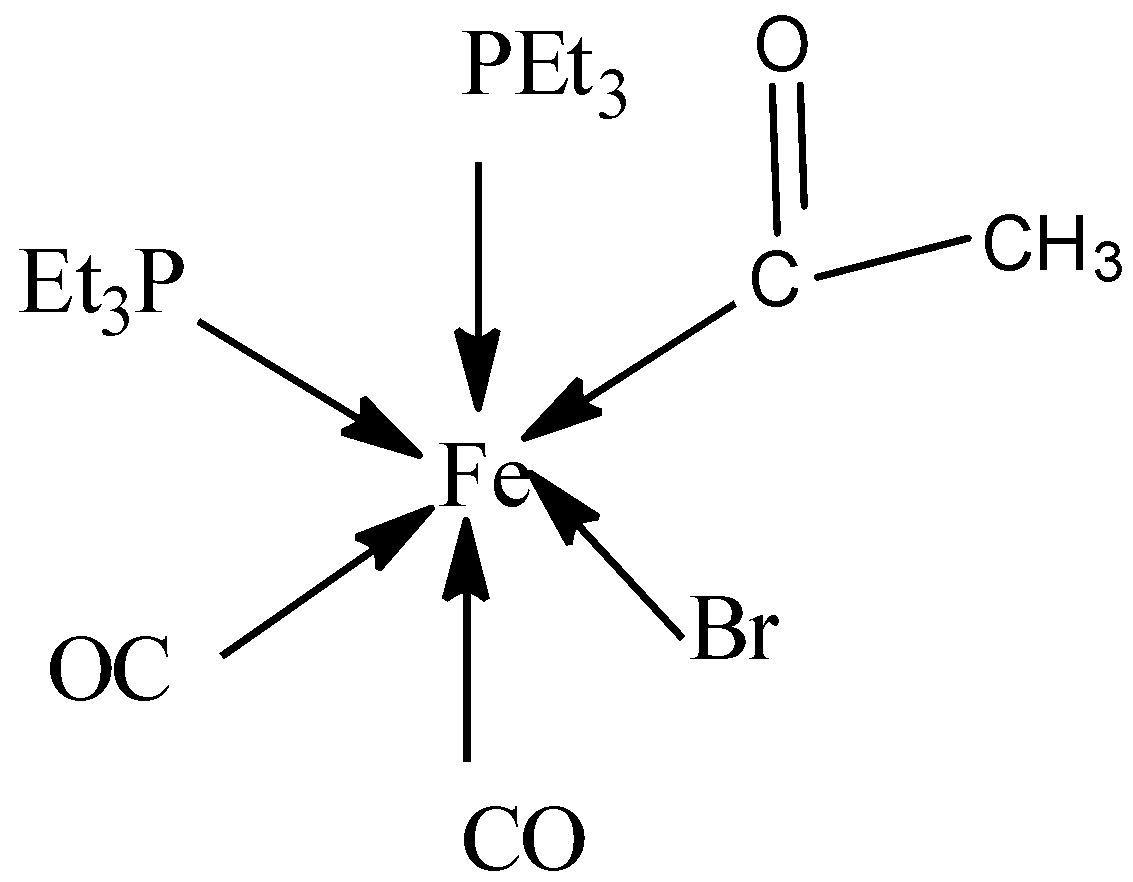
- In the above structure, there are six ligands present in the complex and therefore, the given complex is octahedral.
- Let’s now have a look at the ligands.
- Acetyl ligand is CH3CO−group in which carbon atoms are bonded to the metal forming Fe−C bond.
- Bromido ligand is a bromine atom bonded to the central metal atom.
- Carbonyl ligands are two carbonyl ligands, CO which are bonded to the metal forming twoFe−C bonds.
-bis (triethylphosphine) is the ligand that is having two P(C2H5)3 ligands in which phosphorus atom forms bond with the central metal atom.
- Therefore, out of six ligands bound to iron, three ligands are attached to iron by metal-carbon bond, that is, Fe−C bond.
- Therefore, in the complex acetyl bromide dicarbonyl bis (triethylphosphine) iron (II), the number of Fe−C bonds are three.
Note:
Don’t get confused because of the presence of acetyl groups. In acetyl groups, carbon will be bonded to the metal and not the oxygen atom. Try the method of segregating individual ligands and then find their donor atoms. Then find the type of bond formed with the metal atom to get the answer. Another method could be drawing the structure of the complex and then following the same steps.
