Question
Question: In the Cannizaro reaction given above the slowest step is: 
a) The attack of OH− at the carbonyl group
b) The transfer of hydride to carbonyl group
c) The abstraction of proton from carboxylic acid
d) Deprotonation of PhCH2OH
Solution
Hint : Cannizaro reactions are given by those aldehydes which do not have α hydrogen in their structure. Hydride transfer takes place in the mechanism of Cannizaro reaction which is the slowest step and also the rate determining.
Complete Step By Step Answer:
Cannizaro reaction is the method where in presence of an alkali like sodium hydroxide, the aldehydes having no α hydrogen undergo a disproportionation reaction. A disproportionation reaction here means an intermolecular oxidation and reduction.
As we know aldehydes on oxidation gives carboxylic acid and on reduction form alcohols, so the product formed in the Cannizaro reaction will be a carboxylic acid and an alcohol of the same given aldehyde.
This is a characteristic reaction in case of aromatic aldehydes as they do not have α hydrogen for example, Benzaldehyde.
In the alkaline medium, aromatic aldehydes thus undergo intermolecular oxidation and reduction, forming alcohols and salts of aromatic carboxylic acid as the final product.
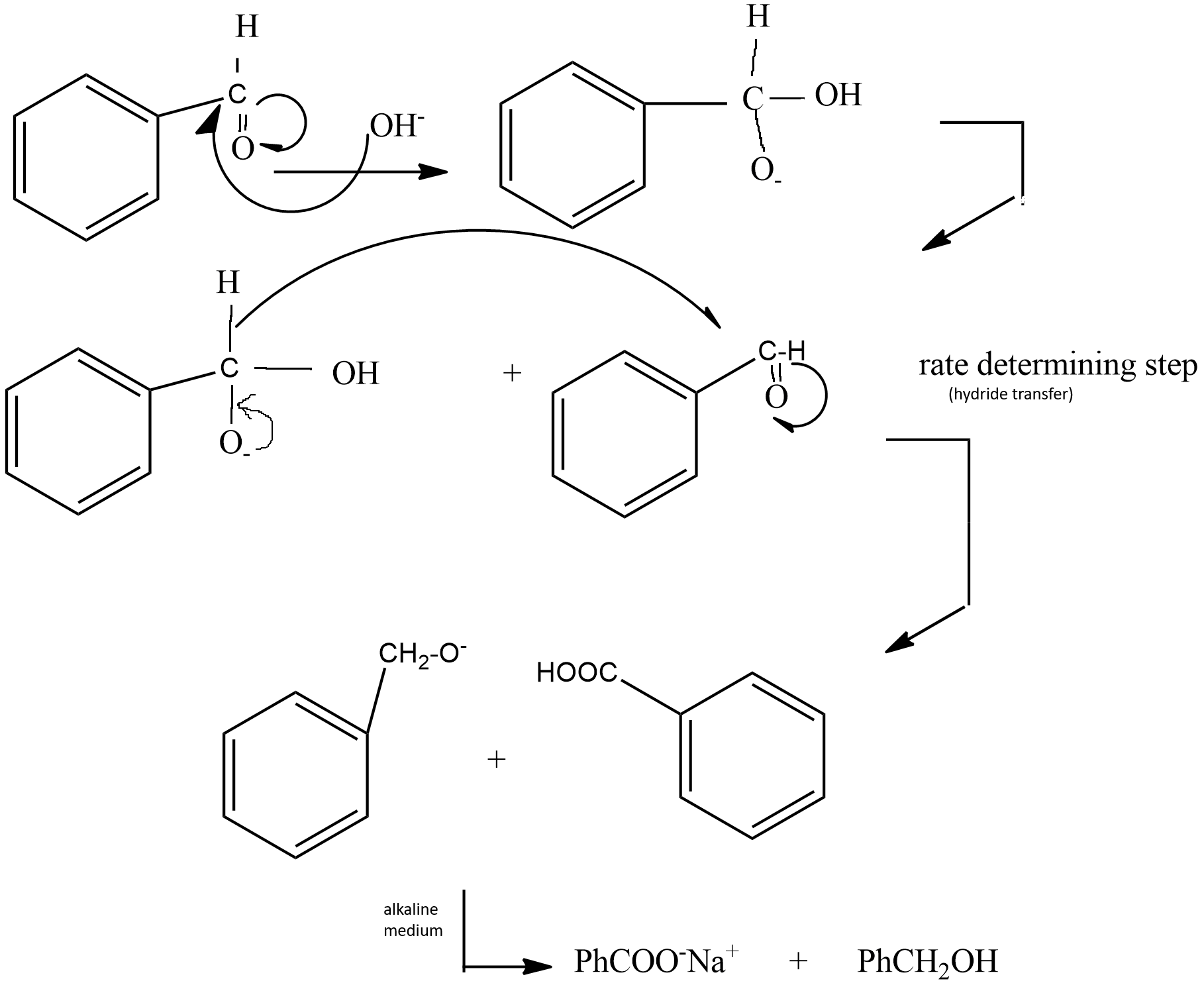
Hence we can list the steps of mechanism as following -
(i) The base present in the medium attacks the carbonyl carbon of aromatic aldehyde, shifting the double bond towards oxygen.
(ii) Then a hydride transfer occurs from the above observed product to another Benzaldehyde molecule. This process is the slowest and rate determining step as breaking of C−H bond occurs with the transfer of hydride to other molecules.
(iii) Then as the medium is alkaline sodium salt of acid is formed.
Like cross Aldol, cross Cannizaro reaction can also occur by reacting aromatic aldehydes with formaldehyde.
Hence option (b) is correct.
Note :
In the cross Cannizaro reaction where an aromatic aldehyde reacts with formaldehyde in an alkaline medium, the conversion of aromatic aldehyde to benzyl alcohol and formaldehyde to formic acid takes place.
