Question
Question: In the below reaction, the reactivity of alcohols is; \(ROH + HX \to RX + {H_2}O\) ; A) \[Terti...
In the below reaction, the reactivity of alcohols is;
ROH+HX→RX+H2O ;
A) Tertiary > secondary > primary
B) Tertiary < secondary < primary
C) Tertiary > primary > secondary
D) Secondary > primary > tertiary
Solution
Alcohols are compounds in which a hydroxyl (– OH) group is attached to saturated carbon atom.
General Formula: R−OH
Monohydric alcohols are classified as primary (1°) secondary (2°), or tertiary (3°), depending upon whether the –OH group is attached to a primary, a secondary, or a tertiary carbon.
Complete step by step answer: Alcohols are reactive compounds. They are attacked by polar or ionic reagents. This is because:
The C–O and O–H bonds of the alcohols are polar since oxygen is highly electronegative.
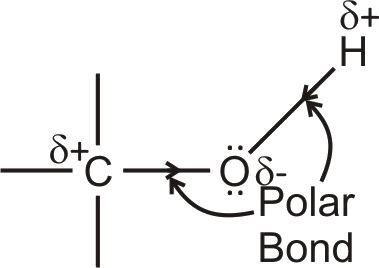
They oxygen atoms of alcohols is an electron-rich centre because it has two unshared pairs of electrons
Alcohols react with hydrogen (HX) to form the corresponding alkyl halides
Eg; CH3CH2OH+HBr→CH3CH2Br+H2O
In general, tertiary alcohols react rapidly with hydrogen halides; secondary alcohols react somewhat slower; and primary alcohols, even more slowly.
The reaction is acid catalyzed. Alcohols react with the strongly acidic hydrogen halides HCl, HBr , and {\text{ }}HI], but they do not react with non acidic NaCl, NaBr, or NaI . Primary and secondary alcohols can be converted to alkyl chlorides and bromides by allowing them to react with a mixture of a sodium halide and sulfuric acid:
The order of reactivity of alcohols is 3∘>2∘>1∘ methyl.
So,Option “A” is correct.
Note: The order of reactivity of hydrogen halidesis HI > HBr > HCl . HCl reacts only in the presence of a catalyst anhydrous(ZnCl2) .No catalyst is required in the case of HBr or HI.
Primary alcohols react with hydrogen halides by an SN1 mechanism.
