Question
Question: In the absence of specific data, it can only be said that (R)-2-bromopentane is: A. Optically inac...
In the absence of specific data, it can only be said that (R)-2-bromopentane is:
A. Optically inactive
B. Analogous in absolute configuration to (R)-2-chloropentane
C. Achiral
D. Dextrorotatory (+)
Solution
We can say an atom that is bonded to four non-equivalent atoms or groups is called the chiral atom. The molecule optically active is due to the asymmetric center.
Complete step by step answer:
We can say that optical activity is the capability of a chiral molecule to rotate plane polarized light.
Asymmetric carbon atom is a tetrahedral carbon atom that is attached to four different atoms or groups known as an asymmetric or chiral carbon atom. It is indicated by an asterisk (∗) on. So, carbon atom is attached to four different groups
An example of a molecule which has an asymmetric carbon is an amino acid. This is because these molecules have a central carbon atom linked to an amino group, carboxyl group, hydrogen atom and a variable side chain.
We can define optically inactive as a compound that is incapable of optical rotation is said to be optically inactive. All pure achiral compounds are optically inactive. Chloroethane is an example of achiral compound and does not rotate the plane of plane-polarized light.
We can define a molecule as chiral if it is superimposable on its mirror image. Most achiral molecules contain a plane of symmetry or a center of symmetry.
We know that there is one chiral carbon is (R)-2-bromopentane. The presence of one chiral carbon in (R)-2-bromopentane makes it optically active.
When the specific data is absent, we can say that (R)-2-bromopentane is related in absolute configuration of (R)-2-chloropentane. Option (B) is correct.
The structures of (R)-2-bromopentane and (R)-2-chloropentane are,
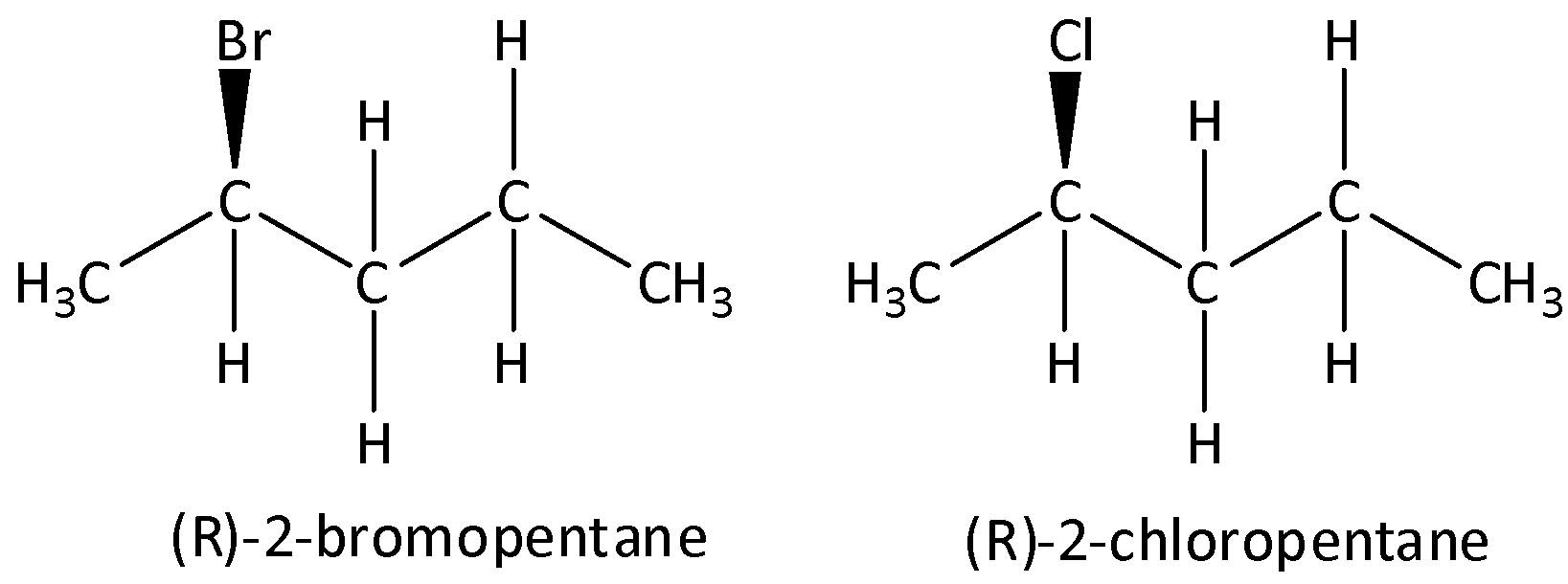
So, the correct answer is Option B .
Note:
We can say that 2-bromopentane is a colorless to yellow-colored liquid with a strong odor. It is less dense than water and is not water soluble. The vapours of 2-bromopentane are heavier than air. It causes irritation in skin and eyes. The molar mass of 2-bromopentane is 151.04g/mol and its chemical formula is C5H11Br.
