Question
Question: In \({\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}^{{\text{2 - }}}\) every \({\text{Cr}}\...
In Cr2O72 - every Cr is linked to:
A) two O-atoms
B) three O-atoms
C) four O-atoms
D) five O-atoms
Solution
Dichromate ion has a chemical formula Cr2O72 - . Draw the structure of the dichromate ion and determine the number of an oxygen atom bonded with each Cr.
Complete step by step answer:
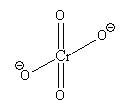
The prefix di stands for 2. So, the ion containing two chromate ions is known as dichromate ion. The structure of chromate ion (CrO42 - ) is as follows:
In chromate ion, there is one Cr atom bonded with 4 O atoms.
The structure of the dichromate ion Cr2O72 - is as follows:
In dichromate ion, there are 2 central Cr atoms and 7 O atoms.
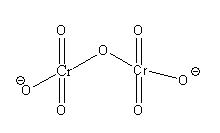
From the structure of dichromate ions, we can say that each central Cr is bonded with 3 terminal oxygen atoms and 1 bride oxygen atom. Out of 7 oxygen atoms, 4 oxygen atoms are bonded with a double bond and 3 oxygen atoms are bonded with a single bond. Thus, each Cr atom in dichromate ion is bonded with 4 oxygen atoms.
Hence, the correct option is (C) four O-atoms.
Additional Information: The bond angle of Cr-O-Cr is 126∘. The bond length of bridge Cr-O is 179pm while the bond length of terminal Cr-O is 163 pm. The bond length of bridge Cr-O is greater than the bond length of the terminal Cr-O. This indicates all Cr-O bonds are not equal. The oxidation state of each Cr atom in dichromate ion is +6.
Note: Two tetrahedral chromate units share the oxygen atom and form dichromate ions. So, the number of oxygen atoms bonded with each Cr atom in dichromate ion is the same as the number of oxygen atoms bonded with Cr atom in chromate ion.
