Question
Question: In \(\text{ Br}{{\text{F}}_{\text{3}}}\) molecule, the lone pairs of electron occupy an equatorial p...
In BrF3 molecule, the lone pairs of electron occupy an equatorial position to minimize
A) Lone pair-bond pair repulsion only
B) Bond pair-bond pair repulsion only
C) Lone pair-lone pair repulsion and lone pair-bond pair repulsion
D) Lone pair-lone pair repulsion only
Solution
In BrF3, there are three bonding pairs Br-F and two lone pairs on the bromine atom.to avoid the repulsion, the lone pairs accommodate the two equatorial position, and the bond pair occupies the three-position, such that it reduces the repulsion between the lone pair’s lone pair and well as between the lone pair and bond pair repulsion.
Complete step by step answer:
Let’s first determine the hybridization of BrF3. The bromine is the central atom and its electronic configuration is as follows;
1s2 !! !! 2s22p6 !! !! 3s23p63d104s24p5
The bromine forms the interhalogen compound with the fluorine. Fluorine can form bonds with the bromine some of the electrons of the bromine are shifted into the 4d− orbit. Fluorine has a higher oxidative capacity and thus it forces the bromine to promote the electron to a higher level.
Now, the bromine uses its d-orbitals to undergo hybridization.
Now, bromine can use the d-orbitals for hybridization.
Br has a total of seven electrons in its outermost shell. On bond formation, three of the electrons are involved in Br−F bonds and 2 lone pairs are left on the bromine. Here , the value of the hybrid orbital is 5, 3 bond pairs, and 2 lone pairs give rise to sp3d hybrid orbitals. The lone pairs also take part in hybridization.
the BrF3 have molecular geometry of T-shaped or trigonal bipyramidal and have a bond angle of 86.20which is slightly less than the usual 900 bond angle. The structure can be
BrF3 molecular geometry is said to be T-shaped or Trigonal Bipyramidal. The lone pairs can be placed on the axial position or equatorial position as shown below With a bond angle of 86.2o which is slightly smaller than the usual 90°.
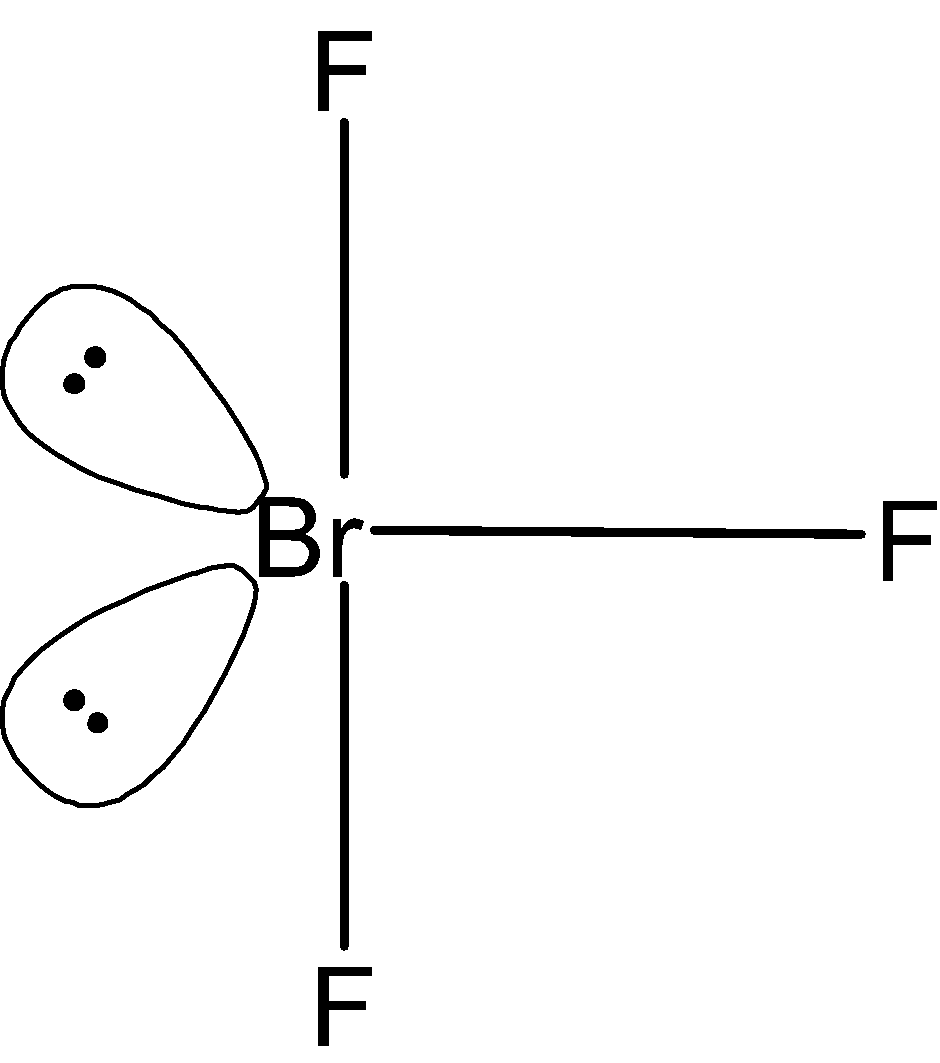
Here, in bent T-shaped geometry, both lone pairs acquire the equatorial position. At the equatorial position, the lone pair experiences the least repulsion. The number of interactions is as follows;
Lone pair-lone pair repulsion is 0.
Lone pair-bond pair repulsion is 4.
Bond pair-bond pair repulsion is 2.
This, stablises the BrF3molecule.
Therefore, in BrF3 a molecule, the lone pairs of electron occupy an equatorial position to minimize lone pair-lone pair repulsion and lone pair-bond pair repulsion.
Hence, (C) is the correct option.
Note: At first glance, BrF3 may look like a trigonal planar structure. But the lone pair on the bromine makes its trigonal bipyramidal. Do not forget that lone pairs near to the atom thus have a higher repulsive nature and distort the geometry.
