Question
Question: In \({\text{B}}{{\text{F}}_{\text{3}}}\), the B-F bond length is 1.30 A. When \({\text{B}}{{\text{F}...
In BF3, the B-F bond length is 1.30 A. When BF3 is allowed to react with Me3N, it forms an adduct, Me3N→BF3. The bond length of B-F in the adduct is:
A. Greater than 1.30 A
B. Smaller than 1.30 A
C. Equal to 1.30 A
D. None of these
Solution
In this question, we will consider the concept of back bonding in the case of BF3. Back bonding happens between the atom having lone pair of an electron, whereas the other atom has a vacant orbital, a pi bond will be formed. Back bonding affects the bond length, but when an adduct is formed there will be no back bonding.
Complete step by step answer:
-First, we will discuss the back bonding in the case of BF3 molecule.
-In this molecule, boron and fluorine both contains an empty p-orbital, whereas the p-orbital of fluorine contains a lone pair of electrons as shown:
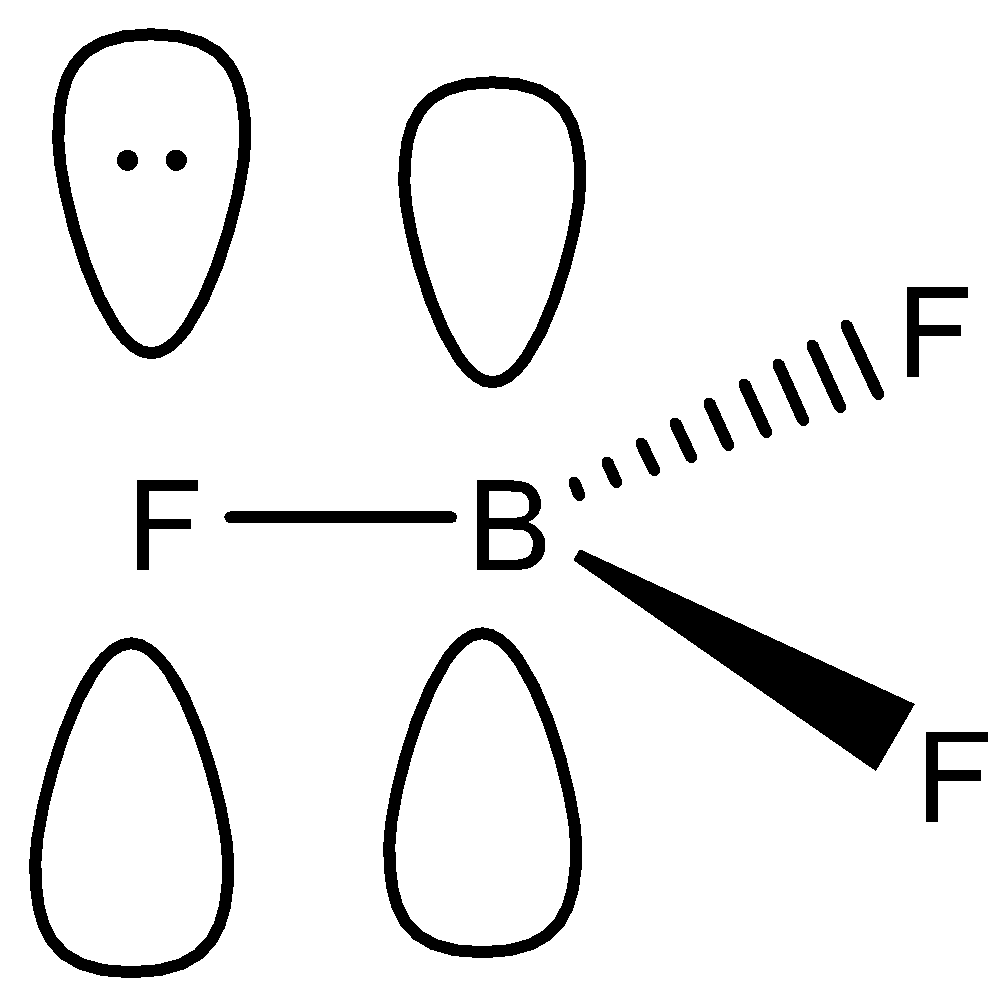
-Now, in this case, fluorine will donate its lone pair to the boron, and there will be the formation of a pi bond as mentioned. We know that this is also known as back bonding.
-If we talk about the bond length, then back bonding leads to the decrease in bond length, but the bond angle remains the same.
-We can say that double bond characteristics are being imparted with back bonding.
-As we know, when BF3 reacts with the Me3N, it forms an adduct.
-Thus, in the adduct, there will be no back bonding, and the double bond characteristics disappear.
-We can say that the no longer presence of back bonding in the molecule will lead to an increase in the bond length.
-So, in the end, we can conclude that the bond length of the B-F bond in the adduct will be greater than 1.30 due to the absence of back bonding.
Hence, the correct option is A.
Note: There could be confusion as to why fluorine donates its lone pair to the boron. Then, we have seen that the fluorine contains a lone pair in its orbital, and it will act as a Lewis base. The other important point is that when an adduct is formed, there will be no pi-bond, it will again form a sigma bond. That’s why the bond length is greater than 1.30 A.
