Question
Question: In \(SO_3^{2 - }\) : A.\(d\pi - p\pi \) bond between \(S\) and \(O\) is delocalized. B.Bonds bet...
In SO32− :
A.dπ−pπ bond between S and O is delocalized.
B.Bonds between S and O are equivalent.
C.There is sp3 hybridized sulphur atom
D.All of the facts given above are true.
Solution
SO32− known as sulphite is a sulphur oxoanion. They are naturally occurring substances present in some foods. The oxidation state of sulphur in sulphite is +4 . Sulphite also shows resonance effect.
Complete step by step answer:
The Lewis structure of SO32− is as follows:
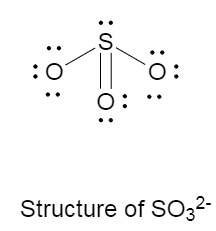
There is one double bond, two single bonds and one lone pair on the central atom that is sulphur.
The molecular structure of sulfite anion is trigonal pyramidal, with bond angle of 109.5∘ .
A.dπ−pπ bond between S and O is delocalized.
Reason: The d-orbital of sulphur overlaps with the p-orbital of oxygen in order to form dπ−pπ bond.
Therefore the above statement is true.
B.the bonds between sulphur and oxygen are same:
Reason: The bonds between sulphur and oxygen are the same because of the resonance effect.
Resonance effect is the shifting of double bonds.
The resonance effect of sulphite is given as follows in the diagram: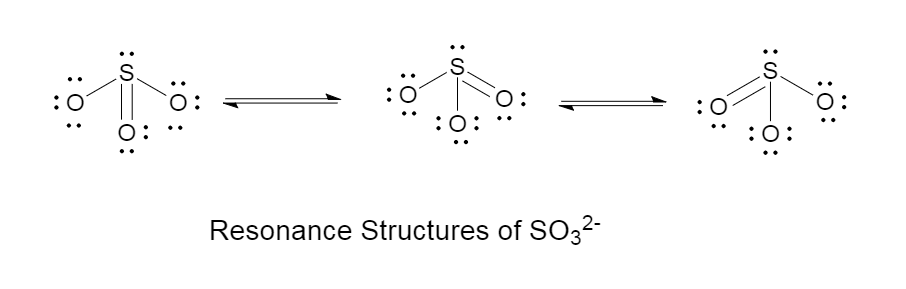
C.There is sp3 hybridized sulphur atom.
In order to check the hybridization of atom we will use the following formula:
Total number of attached atoms + number of lone pairs .
For sp hybridization: Total number of attached atoms + number of lone pair =2
For sp2 hybridization: Total number of attached atoms + number of lone pair =3
For sp3 hybridization: Total number of attached atoms + number of lone pair =4
Now we will calculate the hybridization sulphur in SO32− :
Total number of attached atoms present in sulphur is 3 .
And the number of lone pairs is 1.
⇒ Total number of attached atoms to sulphur + number of lone pair on sulphur
Substituting the values we get,
=3+1
=4
Therefore the hybridization of sulphur in sulphite is sp3 .
Hence the above statement is true.
Therefore all the statements about sulphite are true.
So the correct answer is option D i.e all of the above.
So the appropriate option would be option C.
Note: Due to the presence of lone pairs of electrons on sulphur, they try to push the bonds thus causing the structure to be trigonal pyramidal. Sulfite ion is the conjugate base of bisulphite.
