Question
Question: In \[SN{F_3}\] , the \[\angle FSF\] should be: (a) Less than \[120^\circ \] and more than \[109^\c...
In SNF3 , the ∠FSF should be:
(a) Less than 120∘ and more than 109∘28′
(b) Less than 109∘5′
(c) 180∘
(d) 120∘
Solution
First we have to know, what is the number of valence electrons of the central atom, then the geometry of the given compound and what is the structure of the compound. If the structure and Geometry are not the same then find out the reason why the structure is not as same as the geometry.NSF3 exists as a colourless gas.
Complete answer:
Now, in the NSF3 ,
The central atom is sulphur, whose atomic number is 16 . Hence, the valence electron will be 6 .
(Valence electron: the no. of electrons present in the valence shell are called valence electrons.)
So, the geometry of the will be tetrahedral as the four bonds are present, one triple bond with the nitrogen and three single bonds with fluorine with completing the valency.
The structure of the compound is
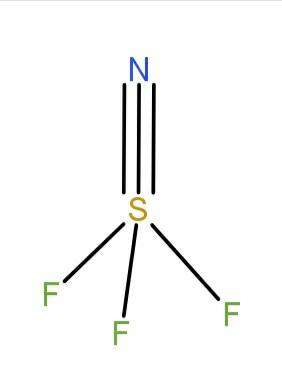
As the fluorine is more electronegative than the nitrogen, the bond-bond repulsion of the compound is not equal. The S−N bonds repels the three S−F more than the S−F repels each other’s bond.
The geometry of the compound is distorted tetrahedral due to the electronegativity difference. Hence, the angle should be smaller than the normal tetrahedral angle (Normal Tetrahedral angle is 109∘28′ ) i.e, the angle should be less than 109∘5′ .
Hence, the correct option of this is (b) Less than 109∘5′ .
Note:
As if the geometry is not same due to the presence of lone pairs, the greater the number of the lone pairs on the central atom, greater the repulsion between them and the lone pairs leads to shortening of the other bond angles to stabilise.
