Question
Question: In \( (S{i_2}{O_5})_n^{2n - } \) anion is obtained when: (A) No oxygen of a \( SiO_4^{4 - } \) tet...
In (Si2O5)n2n− anion is obtained when:
(A) No oxygen of a SiO44− tetrahedron is shared with another SiO44− tetrahedron.
(B) one oxygen of a SiO44− tetrahedron is shared with another SiO44− tetrahedron.
(C) two oxygen of a SiO44− tetrahedron is shared with another SiO44− tetrahedron.
(D) three oxygen of a SiO44− tetrahedron is shared with another SiO44− tetrahedron.
Solution
We need the concept of silicates here. Also, the different types of silicates should be known. It is a part of inorganic chemistry. Depending on the type of bonds, there are seven silicate types.
Complete step by step answer:
We already know the formula: (Si2O5)n2n− .
Silicates are the minerals containing silicon and oxygen in tetrahedral SiO44− units, which are linked together in several patterns. Depending on the way the tetrahedral units are linked, the silicates are classified into the seven types.
The following structure is given below:
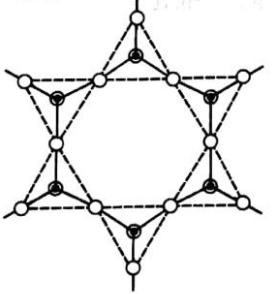
The general formula of Sheet or Phyllo or two dimensional (2-D) silicates is (Si2O5)n2n− . Each SiO4 tetrahedron shares three oxygen atoms with others and thus by forming two-dimensional sheets. These silicates can be cleaved easily just like graphite. The layers are held together by weak van der Waals forces.
Total no. of oxygen atoms per silicon atom = 21+21+21+1=25=2.5
So, the formula comes out to be Si2O5−2
Now we need to select the correct option.
Thus, the correct option is D.
Note:
Silicate minerals are very common in the Earth crust since Oxygen and Silicon are the most abundant elements. The degree of polymerization is denoted by Oxygen to Silicon ratio (O/Si). Greater the degree of polymerization, lower will be the O/Si ratio. With increase in the degree of polymerization, there is decrease in the charge per silicon atom as well as the basicity of silicate minerals. The basic silicate minerals readily react with weak acids and undergo weathering.
Some examples of sheet or Phyllo or two dimensional (2-D) silicates are talc and micas. Talc is the main ingredient of soap stone. Itis the softest material with a smooth and greasy touch.
