Question
Question: In Reimer- Tiemann reaction, reactants are: A.Phenol, \(CHC{l_3}\) and alkali B.Phenol, \(CC{l_4...
In Reimer- Tiemann reaction, reactants are:
A.Phenol, CHCl3 and alkali
B.Phenol, CCl4 and alkali
C.Aniline, CHCl3 and alkali
D.Both phenol, CHCl3 and alkali and phenol CCl4 and alkali
Solution
To solve this question we need to know what Reimer- Tiemann reaction is. It is a type of substitution reaction used for the ortho-formylation of C6H5OH i.e. phenols. Since hydroxides are not readily soluble in chloroform, a biphasic solvent system is employed to carry out this reaction.
Complete step by step answer:
The Reimer Tiemann reaction is named after the scientist Karl Riemann and Ferdinand Tiemann. In this reaction, when phenol is treated with CHCl3 in presence of NaOH and an aldehyde group is introduced at the ortho position of benzene ring leading to the formation of o-hydroxybenzaldehyde.
Now, in this reaction the reactants are both Phenol, CHCl3 and alkali and phenol, CCl4 and alkali. Basically, when phenol, CHCl3 and alkali are used, the product is salicylaldehyde whereas when phenol, CCl4 and alkali are used, the product is salicylic acid. The reactions are as shown:
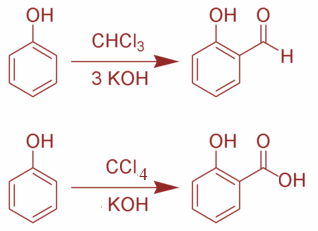
Moreover, the reaction is quite effective when other hydroxyl-aromatic compounds are used such as naphthol. The heterocyclic compounds that are quite rich in electrons such as pyrroles and indoles also undergo this reaction. This reaction is prone to thermal runaways. This is due to the fact that once the reaction starts, it can prove to be highly exothermic and the reaction rate is also increased.
Hence, option D is correct.
Note: However, the direct formulation of aromatic compounds can be accomplished by various methods such as Gattermann reaction, Duff reaction and many more. But in terms of ease and safety of operations, the Reimer-Tiemann reaction is often considered as the most advantageous route in chemical synthesis.
