Question
Question: In reaction, \[Ph-NH-N{{H}_{2}}+(X)+(Y)\xrightarrow{-{{H}_{2}}O}P+Q+S\] Where P is 1-(2-methyl...
In reaction,
Ph−NH−NH2+(X)+(Y)−H2OP+Q+S
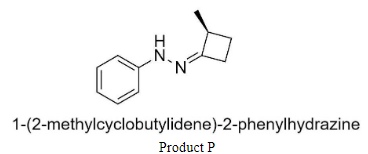
Where P is 1-(2-methylcyclo butylidene)-2-phenylhydrazine,
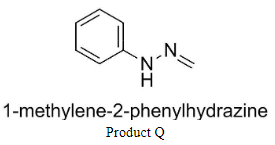
Q is 1-methylene-2-phenylhydrazine and
S is 2-methyl-4-methylene cyclobutanone.
The compounds X and Y can be?
A.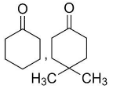
B.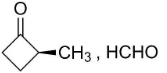
C. HCHO, CH3−CO−CH3
D.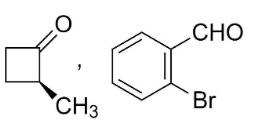
Solution
Products P and Q are examples of hydrazone compounds. Now, hydrazone is a functional group which have structural formula R1R2C=NNH2. Hydrazones are formed by the addition of aldehyde or ketones to hydrazines.
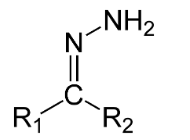
Complete step-by-step answer: We know that the products P and Q are hydrazones having structures:
Now, a hydrazone is formed when the =O a functional group of an aldehyde or a ketone is replaced by −NNH2 functional group of hydrazine. This is a type of condensation reaction which is usually accompanied by the elimination of water.
We can see that in the given question, addition of compounds X and Y to phenyl hydrazine gives products P, Q and S.
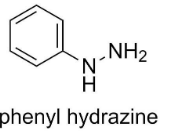
The structure of phenyl hydrazine is
By simply looking at the structures of product P and product Q, we can determine which aldehyde or ketone is added to phenyl hydrazine to form the desired compound.
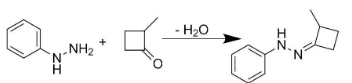
Hence to form product P, we would have to add 2-methyl-4-methylene cyclobutanone to phenyl hydrazine.
Similarly, the addition of formaldehyde to phenyl hydrazine would result in the production of compound Q.
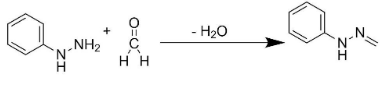
Also, the addition of X and Y would lead to the formation of product S. The entire reaction can be written as follows:
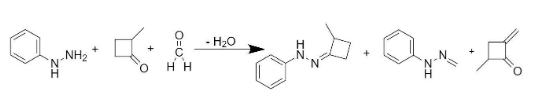
So, the compounds X and Y are option (B)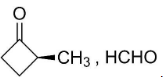
Note: Phenyl hydrazines, when used in access, also react with reducing sugars at boiling temperatures to produce hydrazones. These resultant hydrazones are known as osazones. Osazones can be used to differentiate and identify monosaccharides.
