Question
Question: In pyrophosphoric acid, \({{H}_{4}}{{P}_{2}}{{O}_{7}}\), number of \(\sigma -\) and \(d\pi -p\pi \) ...
In pyrophosphoric acid, H4P2O7, number of σ− and dπ−pπ bonds respectively are:
A. 8 and 2
B. 6 and 2
C. 12 and 0
D. 12 and 2
Solution
Draw the structure of the molecule,H4P2O7. Make sure the valencies of all the atoms are satisfied. Remember that P makes 5 bonds with the surrounding atoms, O makes 2 bonds, and H makes 1 bond.
Complete answer:
Let us consider the structure of pyrophosphoric acid:
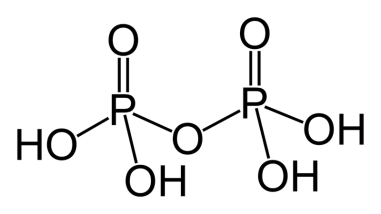
σ−bonds
-Each phosphorus atom is bonded to 2 −OH which makes 4 P−OHsigma bonds.
- Each phosphorus atom is bonded to 2 O atoms which makes 4 P−O sigma bonds.
- Since there are 4 −OH present, 4 O−H sigma bonds are present.
- By adding the number of sigma bonds in these three points we see that the total number of sigma bonds is 12.
Now onto the dπ−pπbonds
- Each phosphorus atom shares a double bond with one O atom, which makes 2 P=O bonds.
- There are no more double bonds to take into account.
- The number of dπ−pπ present are 2.
Therefore, the answer to this question is D. 12 and 2
Additional Information:
The name pyrophosphoric acid was coined due to the way in which the chemical is formed. When phosphoric acid is heated to red heat, it was found that pyrophosphoric acid is formed. This is also an easier way to memorize the molecular structure of this compound.
Note: Please do not confuse the answer with ‘A. 8 and 2’. It is very easy to overlook the bonds that are involved in the O−H system since these bonds are not shown in most images. Make sure that you have considered all the atoms and what bonds they form before writing down the answer.
