Question
Question: In pressure-volume diagram, the isochoric, isothermal, isobaric and iso-entropic parts respectively ...
In pressure-volume diagram, the isochoric, isothermal, isobaric and iso-entropic parts respectively are:
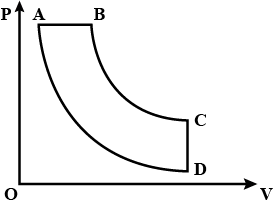
(A) BA,AD,DC,CB
(B) DC,CB,BA,AD
(C) AB,BC,CD,DA
(D) CD,DA,AB,BC
Solution
Since the above given diagram is a pressure-volume curve, we can easily identify all the processes. This can be done by analyzing the graph with respect to the coordinate axes which contains the given variables pressure and volume in the Y and X axis respectively.
Complete answer:
Let us start by analyzing the top of the graph, that is any process from A to B or B to A. Since the line AB or BA is a line parallel to volume and constant for pressure. Thus, AB or BA is an isobaric process.
Similarly, CD or DC being carried out at constant volumes are isochoric processes.
Now, if we move through AD or DA, we can see that it is a rectangular hyperbola. Now, we know at constant temperature, the product of Pressure and Volume is constant and the resultant curves form a rectangular hyperbola. Thus, the process AD or DA is an isothermal process.
And, lastly the remaining curve that is BC or CB must be an iso-entropic process. Now, that we have analyzed the graph, we can check for the correct option.
In only option (D), all the steps are correctly matched to their respective processes.
Hence, option (D) is the correct option.
Note:
In the above graph, we chose AD/DA over BC/CB as an isothermal process because of their slightly more resemblance to a rectangular hyperbola. We cannot truly comment on it without having a proper set of data, but we should always choose the option with more probability that is which seems closer to being the correct choice.
