Question
Question: In presence of U.V. light bromination of benzene produces: A.\({C_6}{H_5}Br\) B.\({C_6}{H_6}B{r_...
In presence of U.V. light bromination of benzene produces:
A.C6H5Br
B.C6H6Br6
C.o & p Dibromobenzene
D.No reaction will be take place
Solution
In the presence of ultraviolet (UV) light or heat, the reaction of a halogen with an alkane results in the formation of a haloalkane (alkyl halide). The phenomenon is explained by the reaction mechanism. The mechanism to halogenate. The carbon-hydrogen bonds are low-polarity covalent bonds in the methane molecule.
Complete answer:
-In presence of U.V. Light bromination of benzene produces C6H6Br6 or 1,2,3,4,5,6-hexachlorocyclohexane. Three molecules of bromine are added across the carbon-carbon triple bond.
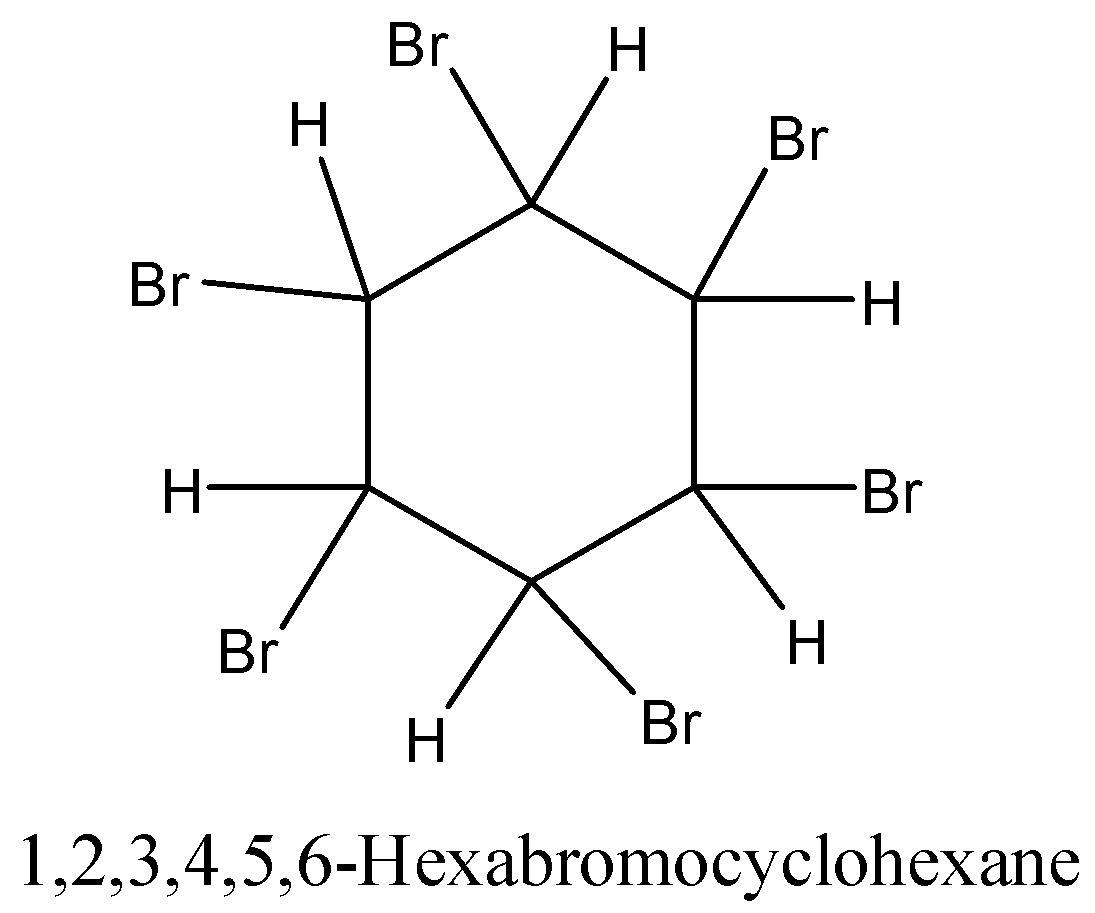
-In the presence of ultraviolet light (but without a catalyst present), hot benzene will also undergo an additional reaction with chlorine or bromine. The ring delocalization is permanently broken and a chlorine or bromine atom adds on to each carbon atom.
-For example, if you bubble chlorine gas through hot benzene exposed to UV light for an hour, you get 1,2,3,4,5,6-hexachlorocyclohexane.
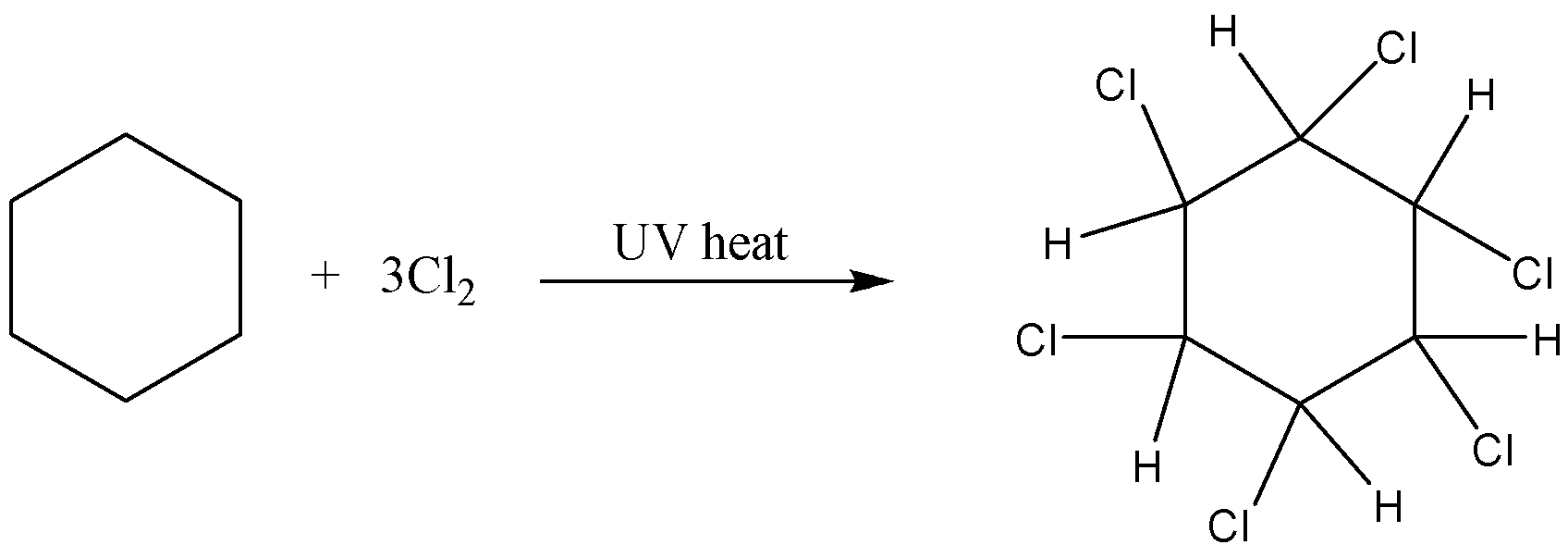
Bromine would behave similarly.
-The bromination of benzene is an example of an electrophilic aromatic substitution reaction. In the reaction, the electrophile (bromine) forms a sigma bond to the benzene ring, yielding an intermediate. Then a proton is removed from the intermediate to form a substituted benzene ring.
Thus, the correct answer is (B).
Note:
-There is no similar reaction between benzene and chlorine in which six chlorine atoms are added around the ring.
-Chlorine adds to benzene in the presence of ultraviolet light. With methylbenzene under those conditions, we get substitution in the methyl group. That is energetically easier because it doesn't involve breaking the delocalized electron system.
-Once all the hydrogens in the methyl group had been substituted, then there might be an addition to the ring as well.
