Question
Question: In phenol carbon atom attached to –OH group undergoes: A. \(s{p^3}\) hybridization B. \(sp\) hyb...
In phenol carbon atom attached to –OH group undergoes:
A. sp3 hybridization
B. sp hybridization
C. sp2 hybridization
D. No hybridization
Solution
We have to know that the formation of a similar number of orbitals containing the same properties of different kinds of orbitals (s&p;) of carbon atom is known as hybridization and orbitals produced are called hybridized orbitals.
Complete step by step answer:
We have to know that sigma bonds are formed by hybrid orbitals of carbon form and those orbitals which do not participate in hybridization to form pi (p) bonds.
A sigma bond is always a single bond. One sigma and one pi bond are found in double bonds. One sigma and two pi bonds are found in triple bonds.
In −C−C bond, the type of hybridization on the carbon atom is sp3.
In >C=C< bond, the type of hybridization on the carbon atom is sp2.
In −C≡C− bond, the type of hybridization on the carbon atom is sp.
We can draw the structure of phenol as,
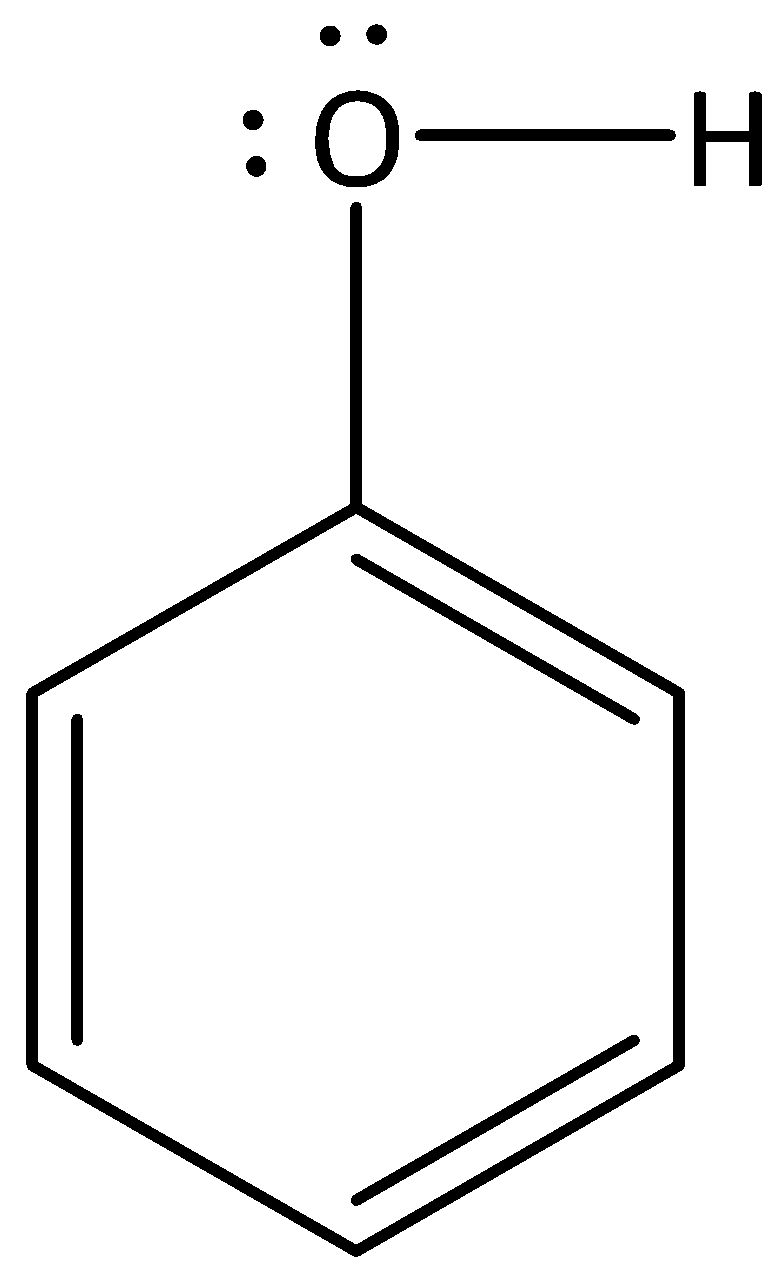
We have to know that in phenol, the carbon to which the hydroxyl group is attached belongs to sp2 hybridization. The increased s-character of the carbon is used to give a C−O bond and makes it a more electron withdrawing group. So, the conjugation with an aromatic ring will make the sp2 hybridized atom of oxygen more likely.
So in phenol, the carbon atom bonded to the hydroxyl group is attached with three sigma bonds and one pi bond. So, the hybridization is sp2.
So option (C) is correct.
Note:
If a compound contains single bonds, then it is sp3 hybridized. Example for sp3 hybridized molecule is methane. Generally, alkanes come under sp3 hybridization. Molecules that have sp3 hybridization will have tetrahedral geometry.
If a compound contains double bonds, then it is sp2 hybridized. Example for sp2 hybridized molecule is ethene. Generally, alkenes come under sp2 hybridization. Molecules that have sp2 hybridization will have trigonal planar geometry.
If a compound contains triple bonds, then it is sp hybridized. Example for sp hybridized molecule is ethyne. Generally, alkynes come under sp hybridization.
