Question
Question: In \( PC{l_5} \) , the three \( P - Cl \) bonds in the plane are called?...
In PCl5 , the three P−Cl bonds in the plane are called?
Solution
Hint : The elements that are present in the third period of periodic table have d orbitals also along with s and p orbitals. The energy of 3d orbitals is close to the energy of 3s and 3p orbitals. The energy of 3d orbitals is also equal to the energy of 4s and 4p orbitals. So, the hybridization is favorable involving either 3d , 3s , 3p or 3d , 4s , 4p .
Complete Step By Step Answer:
The molecular geometry of PCl5 is trigonal bipyramidal. The electron region distribution around the central atom is symmetric. Phosphorus undergoes sp3d hybridization and the geometry will be trigonal bipyramidal. The five valence electrons are shared with one electron each from five chlorine atoms. The structure of PCl5 is given below.
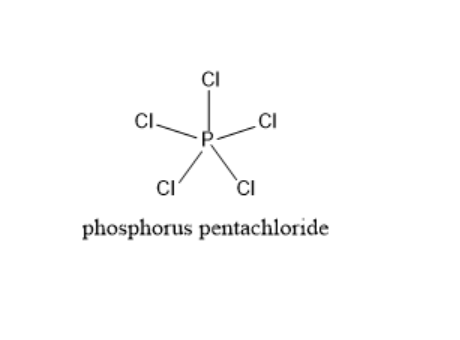
All the P−Cl bonds in the PCl5 molecule are not the same. Two P−Cl bonds are longer and they are less stable. These bonds are called axial P−Cl bonds. The other three P−Cl bonds are more stable and are called equatorial P−Cl bonds. These are the result of greater bond pair-bond pair repulsion in the axial bonds. Phosphorus pentachloride molecules form five bonds in order to expand the octet configuration and to have more place to insert bonding pairs of electrons. The three bonds in the plane are called planar bonds. These are also called equatorial bonds.
Therefore, the three P−Cl bonds in a plane are called equatorial bonds.
Note :
Phosphorus pentachloride is green-yellowish in color and exists as a solid in nature. It has a structure like that of a salt in the crystalline state. It is used as a chlorinating agent and also as a catalyst in the manufacturing of organic chemicals, dyes, etc. It is used as a chlorinating agent. It is used in the pharmaceutical industry in the manufacture of penicillin and cephalosporin.
