Question
Question: In \[PC{l_5}\], the correct statements are, A.It has \(s{p^3}d\) hybridisation with trigonal bi-py...
In PCl5, the correct statements are,
A.It has sp3d hybridisation with trigonal bi-pyramidal geometry.
B.It has three Cl−P−Cl bond angles of 1200 and one of 1800.
C.Axial bond pairs suffer more repulsion from the equatorial bond pairs.
D.Equatorial P−Cl bond are broken during the change PCl5→PCl3+Cl2
Solution
Structure and its related information of PCl5 can be obtained using VSEPR Theory. In VSEPR theory, the hybridisation and structure of a molecule are predicted by counting the number of electron pairs around the central atom.
Complete step by step answer: In PCl5 , the central atom is Phosphorous. It has five valence electrons. We have five chlorine atoms also. Each chlorine atom contains an unpaired electron in p-orbital. The five orbitals of phosphorus containing one electron will thus undergo sp3d hybridisation to give five identical hybrid orbitals. Then each of this orbital will combine with the p-orbital chlorine to form PCl5 . There will be no lone-pair of electrons left on phosphorus. Hence the structure of PCl5 will be trigonal bi-pyramidal. i.e. PCl5 has sp3d hybridisation with trigonal bi-pyramidal geometry. Option A is correct.
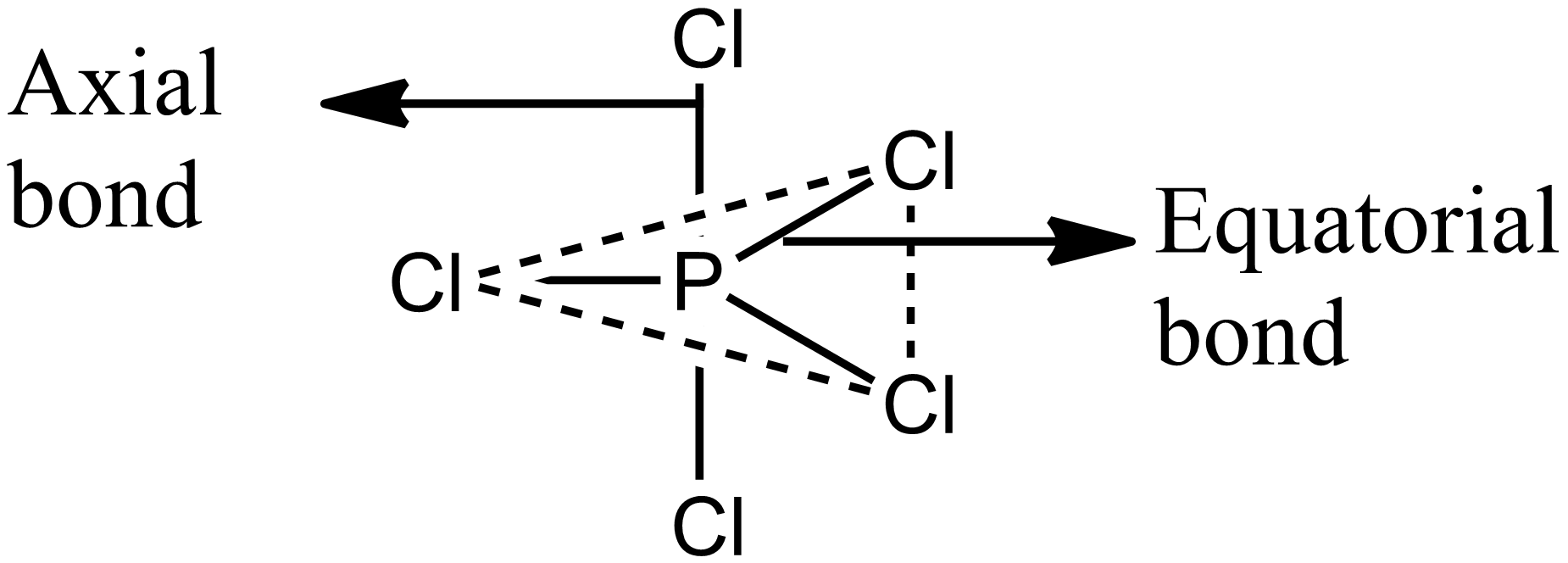
Structure of PCl5 is shown in the figure. It has three equatorial bonds and two axial bonds. The equatorial Cl−P−Cl bond angle is 1200 and axial Cl−P−Cl bond angle is 1800. Hence it has three Cl−P−Cl bond angles of 1200 and one of 1800. Option B is correct.
The angle between equatorial and axial bonds is only 900. Hence bond pair-bond pair repulsion is maximum in axial bonds compared to equatorial bonds. In order to minimize this repulsion axial bonds extend their bond length. Hence axial bonds are more elongated than equatorial bonds. Therefore the option C, axial bond pairs suffer more repulsion from the equatorial bond pairs is correct. Repulsion between equatorial bonds are negligible because their bond angle is 1200.
Since the axial bond suffers more repulsion, it is a weak bond. Hence axial bonds can be broken easily. When PCl5 is heated the axial bonds break to form PCl3 and Cl2. Equatorial bonds do not break. Hence the option D is wrong.
Therefore, the correct options are A,B and C.
Note:
The presence of elongated axial bonds make PCl5 reactive. Here all atoms around phosphorus are Cl. If any of the Cl atom is replaced by another atom, the bond length and bond angles will change. Reactivity of molecules will also change.
