Question
Question: In \( Pbs\left( S \right) + 4{H_2}{O_2}\left( {aq} \right) \to PbS{O_4}\left( s \right) + 4{H_2}O\le...
In Pbs(S)+4H2O2(aq)→PbSO4(s)+4H2O(l) , what was oxidized and what was reduced?
Solution
In a reaction, if one species is getting oxidized and another species is getting reduced then that reaction is called a redox reaction. Find out the oxidation numbers of each of them. An increase in oxidation number means getting oxidized and a decrease in oxidation number means getting reduced.
Complete answer:
The above-given reaction is a redox reaction. One among them is getting oxidized while the other is getting reduced. This can be found out with the help of their oxidation state or number.
The oxidation number is the count of electrons that atoms in a molecule can share, lose or gain while forming chemical bonds with other atoms of a different element. It is also referred to as an oxidation state.
If in a reaction the element is going from a lower oxidation state to a higher oxidation state then it is getting oxidized and if the element is going from a higher oxidation state to a lower oxidation state then it is getting reduced.
Using this concept we will see which among the above is getting oxidized and which is getting reduced.
For the above reaction if we find out the oxidation states then it comes out to be:
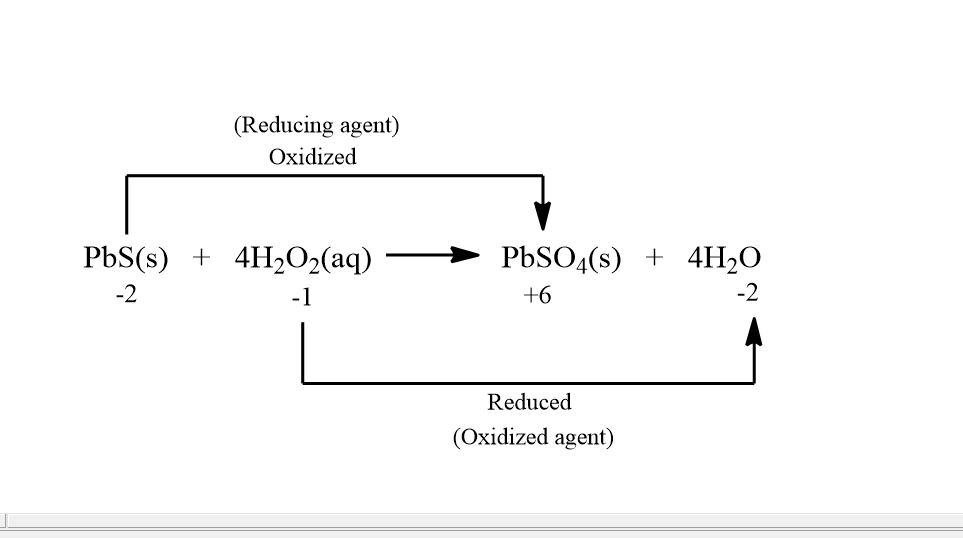
In the above image we can see that in Pbs , s is going from −2 to +6 oxidation state and in H2O2 , O is going from −1 to −2 . And thus Pbs is getting oxidized and H2O2 is getting reduced. Also Pbs is acting as a reducing agent and H2O2 is acting as an oxidizing agent.
Note:
If you want to be precise in your answer then you can say that the oxidized species is S and the reducing species is O . This is because these two elements are undergoing a change in the oxidation states and in the rest of the elements there is no change in the oxidation number. Any species getting oxidized acts as a reducing agent and any species getting reduced acts as an oxidizing agent.
