Question
Question: In \(P{O_4}^{3 - }\), the formal charge on each oxygen atom and \(P - O\) bond order are respectivel...
In PO43−, the formal charge on each oxygen atom and P−O bond order are respectively.
A) −0.75,0.6
B) −0.5,1.0
C) −0.75,1.25
D) −3.0,1.5
Solution
We know that the charges that are assigned to each atom in a molecule or ion by a set of arbitrary rules and do not actually represent the actual charges on the atoms are called as formal charges.
The formal charge is calculated using the formula,
F.C=Valence electrons−No. of non - bonding electrons−2No. of bonding electrons
Complete step by step answer:
We can calculate the bond order of the phosphate ion using the formula,
Bond order=Number of resonating structuresNumber of Bonds
Now, we see the resonating structures of phosphate ion,
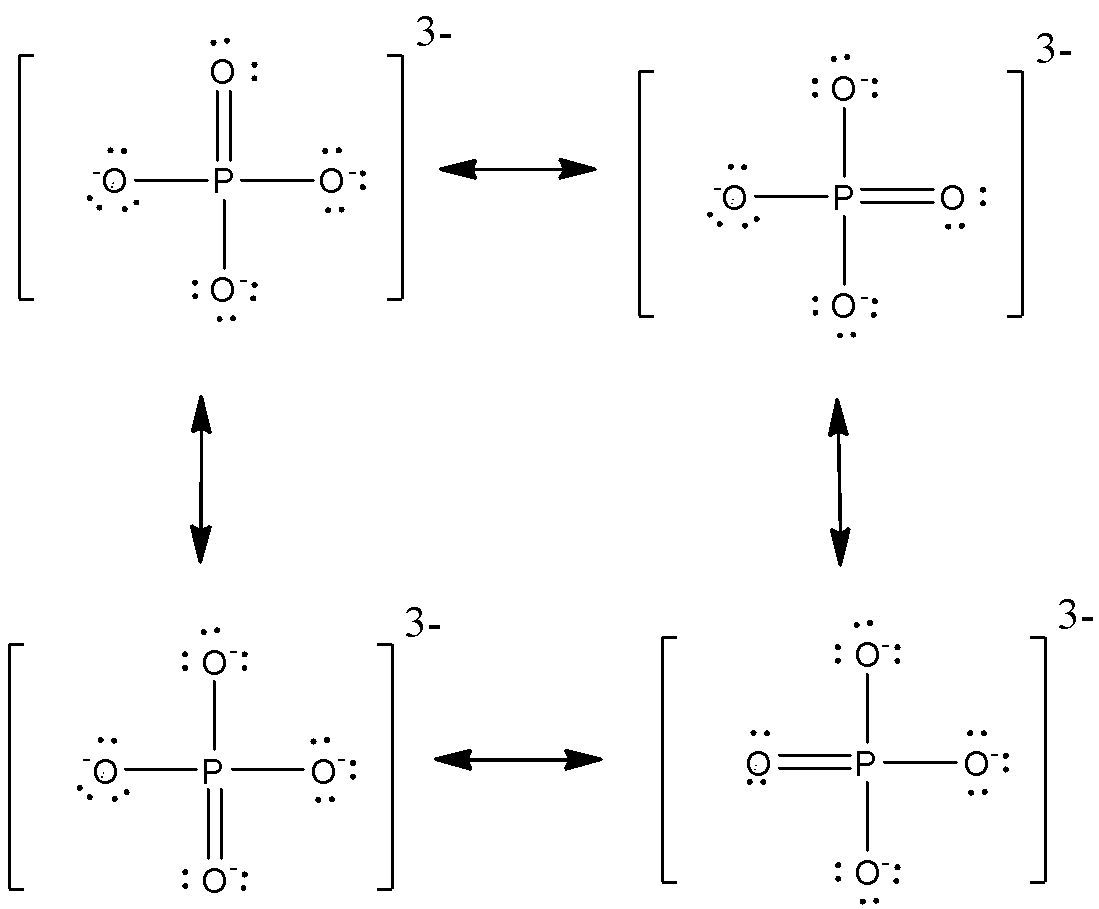
Bond order =45=1.25
To calculate the formal charge, use the above formula.
For oxygen atom that forms double bond with Phosphorus atom,
Formal charge =[6]−[4+2]=0.
For oxygen atom that forms single bond with Phosphorus atom,
Formal charge =[6]−[6 +1]=− 1.
In the resonance structure, a total of −3 charge is distributed over four oxygen atoms. Thus the formal charge of each oxygen atom is 4−3=−0.75.
So, the correct answer is Option C .
Note:
Procedure to write Lewis formulas:
1.The symbols of the atoms that are bonded together in the molecule next to one another are arranged.
2.The total number of valence electrons in the molecule is calculated by adding the number of valence electrons for all the atoms in the molecules. If the species is an ion, then the charge of ions is taken into account by adding electrons, if it is a negative ion or subtracting electrons if it is a positive ion.
3.A two-electron covalent bond is represented by placing a line between the atoms, which are assumed to be bonded to each other.
4.The remaining valence electrons as lone pairs about each atom are arranged so that the octet rule is satisfied for each other.
Resonance: Each of the individual Lewis formulas is said to be a resonance form and the use of multiple Lewis formula is called resonance. The two dashed lines taken jointly represent a pair of bonding electrons to stretch over the two bonds. Such a superimposed formula is called resonance hybrid because it is a hybrid of the various resonance forms.
