Question
Question: In \(P{O_4}^{3 - }\), the formal charge on each oxygen atom, and \({\text{P - O}}\) bond order respe...
In PO43−, the formal charge on each oxygen atom, and P - O bond order respectively are:
(a) −0⋅75,0⋅6
(b) −0⋅75,1⋅0
(c) −0⋅75,1⋅25
(d) −3,1⋅25
Solution
Formal charge calculating equation is a formal way of comparing the total number of valence electron in a neutral, and isolated atom with the number of valence electrons around the atom in the molecule in which it is bonded, and order is the number of bonds between a pair of atoms.
Complete step by step answer:
Step (1): Calculation of formal charge:
Formal charge = (Number of valence electrons in the neutral isolated atom) - (Number of valence electrons around the atom in the bonded state with the molecule)
= (Number of valence electrons in the neutral isolated atom)- 21(Number of shared electrons in the covalent bond between them) -(Number of lone pair of electrons) ..……… (1)
Valence electrons correspond to the group number of the atom in the periodic table.
Lone pair electrons are the group of two electrons in a pair which are left on the atom after the bond formation.
Shared electrons are the electrons which took part in the bond formation. 21 is used in the formula because each bond consists of two electrons, so, each atom can get the credit of one electron only in a bond.
The structure of PO43− will be:
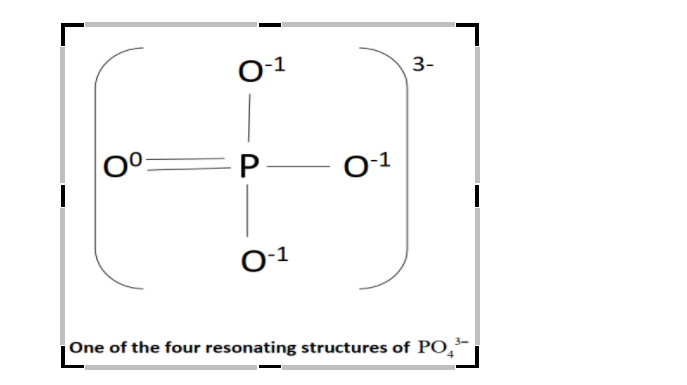
Since, formal charge is the charge assigned to an atom in the molecule, so, the numbers above the atoms signifies their formal charge in the above figure.
Formal charge of O - atomhaving single bond with P - atomfrom equation (1) will be:
= (Number of valence electrons in the O - atom)-21(Number of shared electrons in the covalent bond between O and P)- (Number of lone pair of electrons of O - atom)
= 6−21×2−6
=−1
Formal charge of O - atomhaving single bond with P - atomfrom equation (1) will be:
= 6−21×4−6
= 0
Since, PO43− has 4 resonating structures, so, formal charge will be divided equally between the 3 single bonded O - atoms.
Each atom has charge = - 1. So, 3 atoms have charge = −1×3=−3.
Total number of O - atoms present = 4. So, Formal charge of each O - atom
= Total atomsTotal charge
= 4−3
=−0⋅75
Step (2): Calculation of P - O bond order:
Total number of bonds in PO43− = 5, and Total number of resonating structures of PO43− = 4
Now, Bond order = Total number of resonating structuresTotal number of bonds in the molecule
= 45
= 1⋅25
So, the formal charge on each O - atom is −0⋅75, and P - O bond order is 1⋅25.
So, the correct answer is Option C .
Note:
In the formula of Formal charge, do not write the number of pairs of electrons, instead write the total number of electrons in each pair. Also, the bond order is inversely proportional to the bond length between the atoms in consideration.
