Question
Question: In \(P{{O}^{3-}}\) ion, the effective charge on each oxygen atom and P-O bond order respectively are...
In PO3− ion, the effective charge on each oxygen atom and P-O bond order respectively are-
(A) (-0.75), 1.25
(B) (-0.75), 1.0
(C) (-0.75), 0.6
(D) (-3), 1.25
Solution
Draw all the resonating structures of PO3−ion. Also, Bond order = number of resonating structuresnumber of bonds
Effective charge = total no. of O atomstotal charge on ion
Complete answer:
Firstly, draw all the resonating structures of PO3− ion:
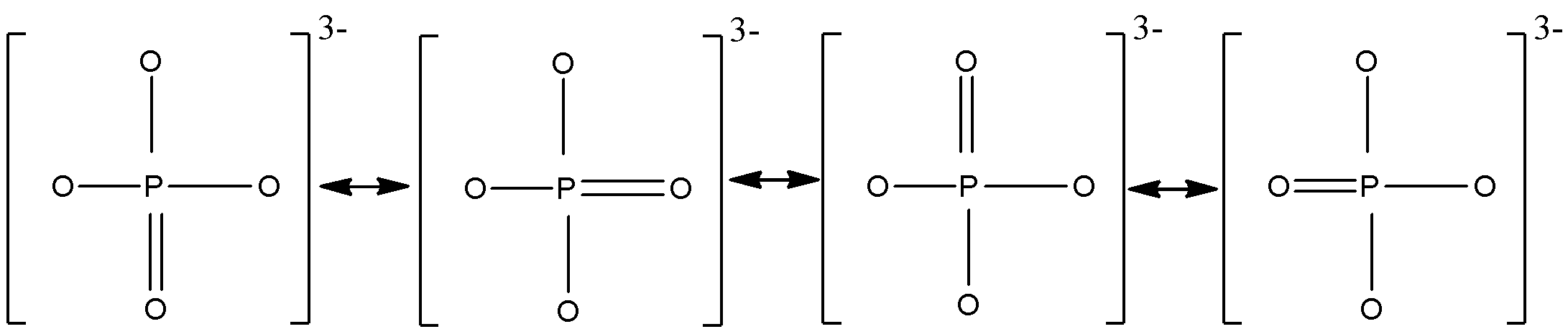
Bond order = number of resonating structuresnumber of bonds
Total number of bonds =5
Number of resonating structures =4
Therefore, Bond order = 45=1.25
Since we know that Effective charge = total no. of O atomstotal charge on ion
In these resonance hybrid structures, a total of -3 charge is distributed over 4 oxygen atoms. Hence, the effective charge of each O atom is 4−3=−0.75
Hence, in PO3− ion, the effective charge on each oxygen atom and P-O bond order respectively are (-0.75), 1.25 respectively.
Therefore, the correct answer is option (A) (-0.75), 1.25
Additional information:
Bond order is the number of chemical bonds between a pair of atoms and depicts the stability of a bond. When bond order is zero, the molecule cannot form. The higher bond orders indicate greater stability for the chemical species. In molecules that have resonance bonding, the bond order may not be an integer. In molecular orbital theory (MOT), bond order is described as half the difference between the no. of bonding electrons and the no. of antibonding electrons.
Note:
Formal charge on each atom can be calculated by the following formula-
FC= No. of valence electrons on atom - unshared electrons - number of bonds
The formal charge on an atom in a molecule is important as it shows the electron count associated with the atom as compared to the isolated neutral atom.
Alternatively, Bond order in this question can be calculated as :Bond order = number of bonding groupsnumber of bonds=45=1.25
