Question
Question: In \({P_4}{O_{10}}\) the: (A) Second bond in \(P = O\) is formed by \(p\pi - d\pi \) back bonding ...
In P4O10 the:
(A) Second bond in P=O is formed by pπ−dπ back bonding
(B) P=O bond is formed by pπ−pπ bonding
(C) P=O bond is formed by dπ−dπ bonding
(D) P=O bond is formed by dπ−dπ−3σ back bonding
Solution
P4O10 (phosphorus Pentoxide) is a chemical compound. This while crystalline solid is the anhydride of phosphoric acid. It is a powerful desiccant and dehydrating agent.
Complete step by step answer:
In P4O10, the terminal P−O bonds are formed by pπ−dπ back bonding. This bonding results in the formation of a coordination bond resulting from an overlap of the p-orbitals of oxygen with empty dπ orbitals of phosphorus. Here, phosphorus atoms donate one pair of electrons resulting in π back bonding.
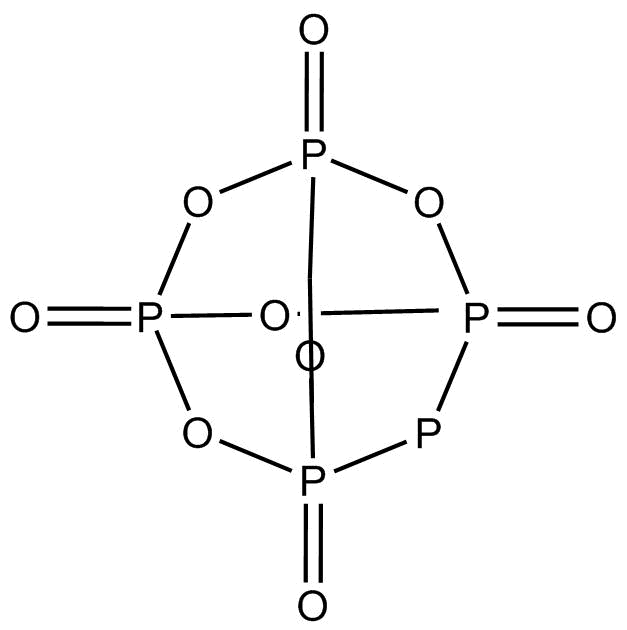
The molecules are held together in a hexagonal lattice by weak Vander Waals forces.
This structure contains 6P−O−P bonds where the hybridization of oxygen is sp3. Another 4 oxygens are attached to each phosphorus by P=O where hybridization of oxygen is sp2. All the 10 oxygen contains 20lone pairs (2 lone pairs each).
Preparation: P4+5O2→P4O10.
Hence, option B is the answer.
Note:
P4O10 is called pentoxide because of the oxidation state of phosphorus in the compound. The name of phosphorus pentoxide itself suggests that the name is related to phosphorus. The naming of any compound is done using its empirical formula. Since, the empirical formula of P4O10 is P2O5, it is called phosphorus pentoxide.
