Question
Question: In \({{P}_{4}}{{O}_{10}}\) molecule, bridging P-O bond length is: A. Larger than that of in \({{P}...
In P4O10 molecule, bridging P-O bond length is:
A. Larger than that of in P4O6
B. lesser than that of in P4O6
C. equal to that of in P4O6
D. cannot be compared
Solution
The presence of double and single bonds leads to the bond length and orientation of bonds in a molecule. P4O10is tetra phosphorus decaoxide. This molecule consists of a double bond character in the oxygen bond.
Complete answer:
We have to find about the P-O bond length in P4O10 molecule and compare it with the P-O bond in P4O6 molecule.
As we know that the multiplicity of bonds affects the bond length. Double bonds and triple bonds tend to be of lesser length than single bonds. This is the reason why some bonds have more bond length and some bonds have less bond length. The presence of any double bond in the oxygen atom in both the oxides of phosphorus will tell us the information about the P-O bridge.
The molecule of P4O10 is as follows:
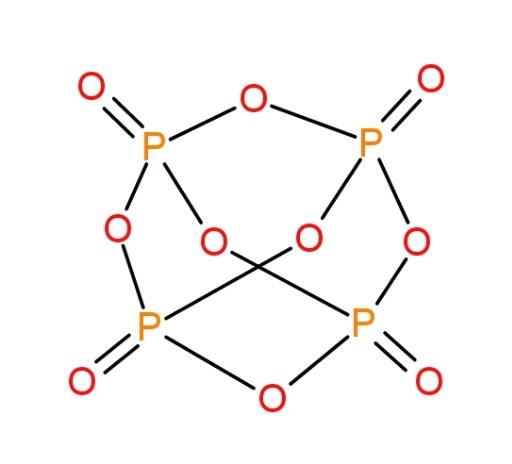
We can see the presence of 4 P-O bonds here. These bonds are double bonds (4) and others single bonds.
Now here is the P4O6 molecule:
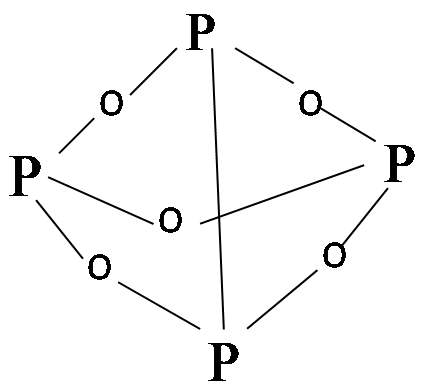
This molecule consists of a network of only single bonds in the P-O bonds.
Clearly, from the nature of P-O bonds present in both the molecules, it can be inferred that P-O bond length in P4O10 is lesser than that of in P4O6 molecule, due to the presence of double bonds.
Hence, P-O bond in P4O10 is lesser than P-O bond in P4O6.
Thus option B is correct.
Note:
From the observed measurements of the P-O bonds in P4O6 and P4O10, the value of P-O bond in P4O6 is 166 pm, while that of this bond in P4O10 is 143 pm. This also proves that P-O bond in P4O6 is larger than P-O bond in P4O10.
