Question
Question: In \[{P_4}{O_{10}}\]find the number of oxygen atoms bonded to each phosphorus atom....
In P4O10find the number of oxygen atoms bonded to each phosphorus atom.
Solution
In the question we know that the phosphorus is bonded to oxygen atoms. The number of oxygen atoms are ten, but it is not necessary that all are bonded to phosphorus. There are only four phosphorus in the given molecule P4O10.
Complete step by step answer:
To know how-many oxygen is bonded to phosphorus let's draw the structure. We have to know how it is formed.
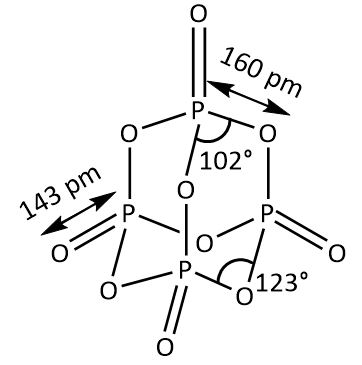
The structure as shown in the above figure contains four phosphorus atoms and ten oxygen atoms. TheP4O10 is formed by the combustion reaction between tetra phosphorus in the presence of oxygen. The reaction is as follows: P4+5O2→P4O10 here the phosphorus is white allotrope of phosphorus.
Another way of formation of P4O10is by combining the two molecules of P2O5. Hence, P4O10is also called a dimer of P2O5. The hexagonal cell of a molecule of phosphorus pentoxide is bonded together by weak van der Waals forces, known as the electrostatic attraction between molecules of P2O5. The Dimer of Phosphorus pentoxide contains 6P−O−P bonds and 4P=O bonds. The dipole-dipole interactions of the P−O−P bonds are what keep the molecule together. P−O−P bonds are polar with a negative valence on the oxygen atom. The empirical formula of P4O10 is P2O5. The relation between the empirical formula and molecular formula is molecular formula is n times empirical formula {P_4}{O_{10}}$$$$ = \;2({P_2}{O_5}). Same as the empirical formula of benzene and methane are carbon and hydrogen CH. It is also known as phosphorus pentoxide, phosphoric anhydride, and tetra phosphorus decaoxide, whereas phosphorus pentoxide is a common name.
Hence, the number of oxygen atoms bonded to each Phosphorus atom is four.
Note:
Do not get confused with the number of oxygen atoms that are bonded to two phosphorus atoms.
