Question
Question: In ozonolysis of benzene, how many molecules of ozone are added?...
In ozonolysis of benzene, how many molecules of ozone are added?
Solution
Ozonolysis is an organic reaction which is used to break the unsaturated bond of alkenes, alkynes or azo compounds. Ozonolysis with one molecule of ozone converts alkenes into carbonyl compounds. Then, after reduction of this carbonyl compound with Zinc metal we get aldehydes. To break a double bond, we need one molecule of ozone gas.
Complete step by step answer:
Ozonolysis with one molecule of ozone converts alkenes into carbonyl compounds. Then, after reduction of this carbonyl compound with Zinc metal we get aldehydes. To break a double bond, we need one molecule of ozone gas.
In benzene there are three double bonds. During ozonolysis of benzene each double bone is converted into a carbonyl group.
Then, after reduction of carbonyl groups we have three molecules of glyoxal.
Here, three alkenes or double bonds are broken during ozonolysis of benzene then we need three molecules of ozone. Each molecule of ozone reacts with a double bond of benzene.
For better understanding of ozonolysis of benzene see the diagram below:
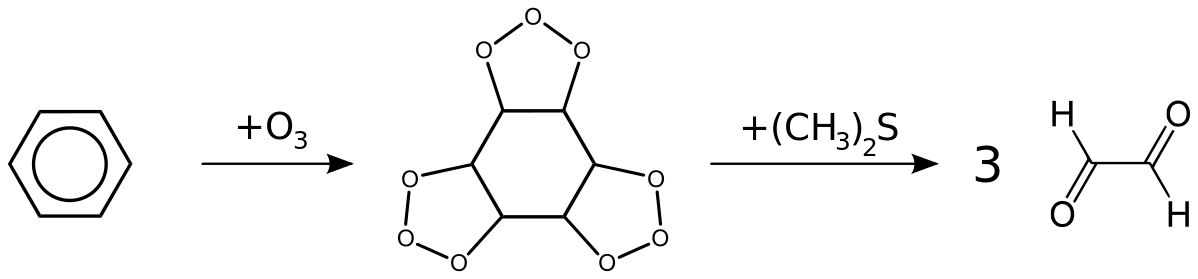
In the above diagram, we easily see that three ozone are reacted with benzene during the ozonolysis of ozone.
Note:
Benzene has three double bonds and when we react benzene with ozone all three double bonds are getting reacted with three molecules of ozone. After this reaction benzene is attached with ozone molecules as shown in the diagram. During reduction in presence of zinc metal oxygen atoms are removed and we get three molecules of glyoxal.
